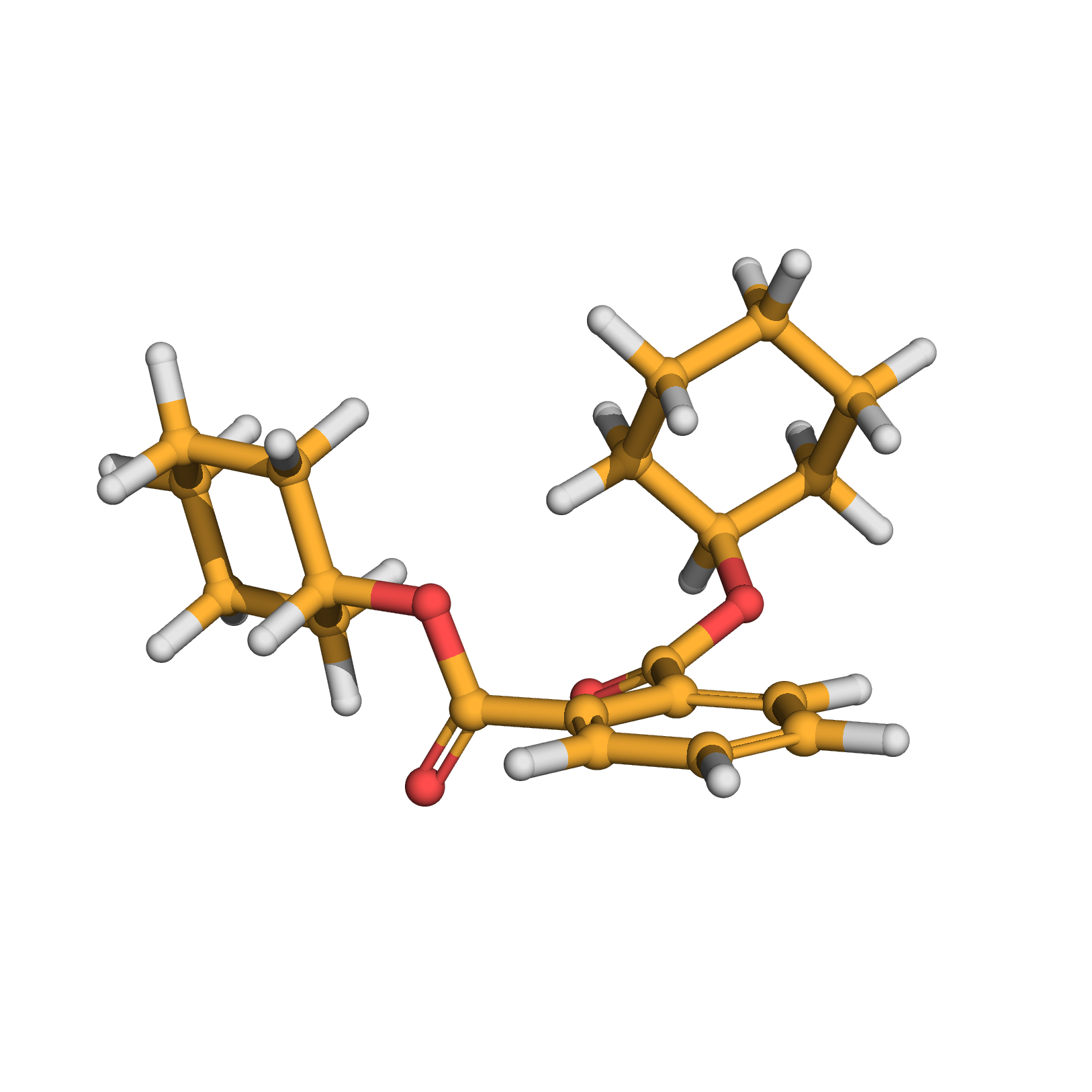
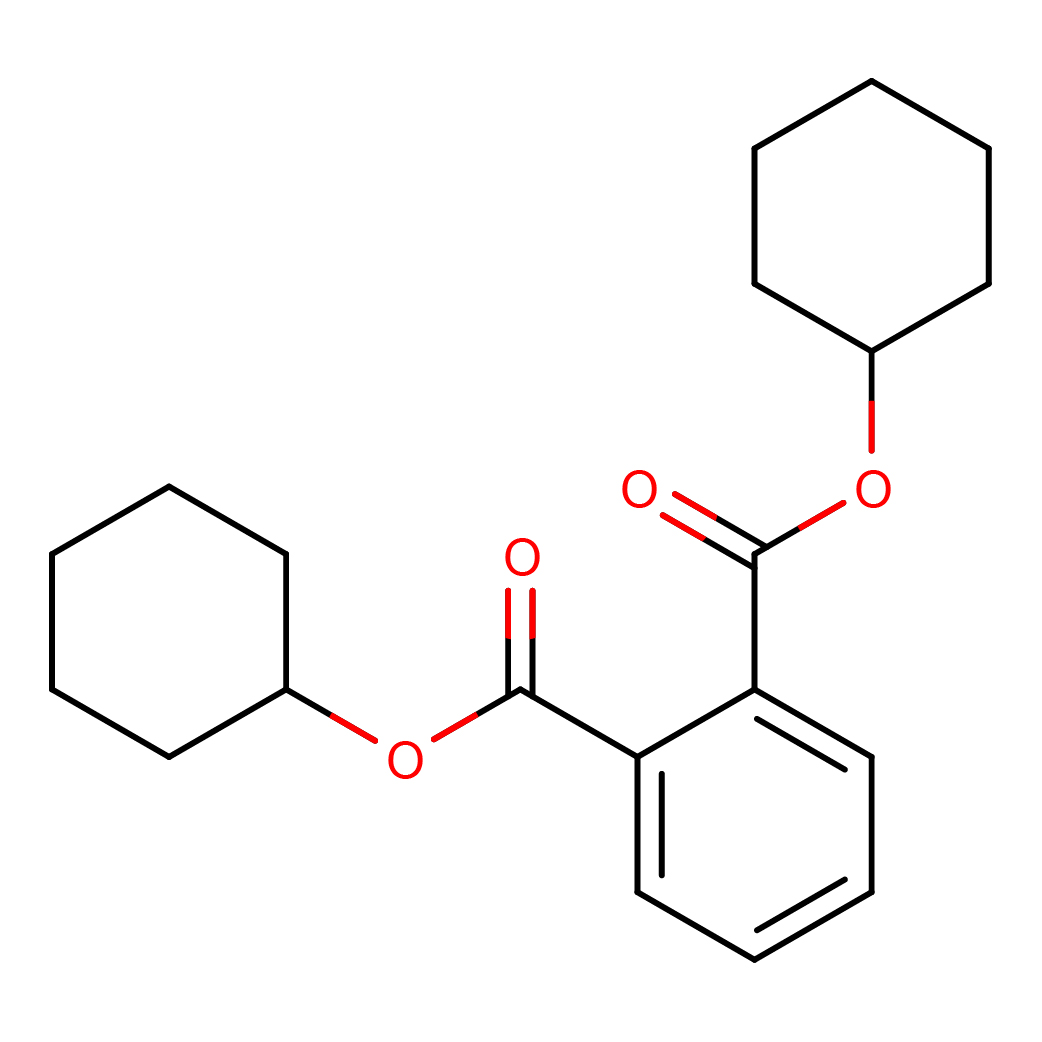
dicyclohexyl phthalate
Synonyms: "ergoplast FDC", "phthalic acid", "dicyclohexyl ester", "1,2-benzenedicarboxylic acid, dicyclohexyl ester"
Source: dicyclohexyl phthalate is a plasticiser with numerous uses. It is found in cellulose and PVC products, in paints, inks and in food packaging.
Identifiers:
IUPAC Name: dicyclohexyl benzene-1,2-dicarboxylate
CAS Number: 84-61-7
PubChem ID: 6777
InChiKey: VOWAEIGWURALJQ-UHFFFAOYSA-N
Canonical SMILES: C1CCC(CC1)OC(=O)C2=CC=CC=C2C(=O)OC3CCCCC3
Structural Properties:
Molecular Formula: C20H26O4
Molecular Weight: 330.418
Pharmacophore Features:
Number of bond donors: 0
Number of bond acceptors: 4
Number of atoms different from hydrogen: 24
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Fan WQ, Yanase T, Morinaga H, Gondo S, Okabe T, Nomura M, Komatsu T, Morohashi K-I, Hayes TB, Takayanagi R, Nawata H. 2007. Atrazine-induced aromatase expression is SF-1 dependent: implications for endocrine disruption in wildlife and reproductive cancers in humans. Environ Health Perspect 115(5):720-727.
Ishido M, Masuo Y, Sayato-Suzuki J, Oka S, Niki E, Morita M. 2004. Dicyclohexylphthalate causes hyperactivity in the rat concomitantly with impairment of tyrosine hydroxylase immunoreactivity. J Neurochem 91(1):69-76.
Okubo T, Suzuki T, Yokoyama Y, Kano K, Kano I. 2003. Estimation of estrogenic and anti-estrogenic activities of some phthalate diesters and monoesters by MCF-7 cell proliferation assay in vitro. Biol Pharm Bull 26(8):1219-1224.
Sargis RM, Johnson DN, Choudhury RA, Brady MJ. 2010. Environmental endocrine disruptors promote adipogenesis in the 3T3-L1 cell line through glucocorticoid receptor activation. Obesity (Silver Spring) 18(7):1283-1288.
Yamasaki K, Okuda H, Takeuchi T, Minobe Y. 2009. Effects of in utero through lactational exposure to dicyclohexyl phthalate and p,p'-DDE in Sprague-Dawley rats. Toxicol Lett 189(1):14-20.
External Links


2D-structure
3D-structure