butyl benzyl phthalate
Synonyms: "butylbenzyl phthalate", "benzyl butyl phthalate", "sicol", "palatinol BB", "unimoll BB", "santicizer 160", "sicol 160"
Source: butyl benzyl phthalate is mostly used as a plasticizer for PVC (polyvinyl chloride).
Identifiers:
IUPAC Name: 2-O-benzyl 1-O-butyl benzene-1,2-dicarboxylate
CAS Number: 85-68-7
PubChem ID: 2347
InChiKey: IRIAEXORFWYRCZ-UHFFFAOYSA-N
Canonical SMILES: CCCCOC(=O)C1=CC=CC=C1C(=O)OCC2=CC=CC=C2
Structural Properties:
Molecular Formula: C19H20O4
Molecular Weight: 312.360
Pharmacophore Features:
Number of bond donors: 0
Number of bond acceptors: 2
Number of atoms different from hydrogen: 23
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Coldham NG, Dave M, Sivapathasundaram S, Mcdonnell DP, Connor C, Sauer MJ. 1997. Evaluation of a recombinant yeast cell estrogen screening assay. Environ Health Perspect 105(7):734-742.
Jobling S, Reynolds T, White R, Parker MG, Sumpter JP. 1995. A variety of environmentally persistent chemicals, including some phthalate plasticizers, are weakly estrogenic. Environ Health Perspect 103(6):582-587.
Kanayama T, Kobayashi N, Mamiya S, Nakanishi T, Nishikawa J. 2005. Organotin compounds promote adipocyte differentiation as agonists of the peroxisome proliferator-activated receptor [gamma]/retinoid X receptor pathway. Mol Pharmacol 67(3):766-774.
Lampen A, Zimnik S, Nau H. 2003. Teratogenic phthalate esters and metabolites activate the nuclear receptors PPARs and induce differentiation of F9 cells. Toxicol Appl Pharmacol 188(1):14-23, DOI: 10.1016/S0041-008X(03)00014-0.
Lu KY, Tseng FW, Wu CJ, Liu PS. 2004. Suppression by phthalates of the calcium signaling of human nicotinic acetylcholine receptors in human neuroblastoma SH-SY5Y cells. Toxicology 200(2-3):113-121.
External Links
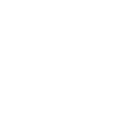

2D-structure
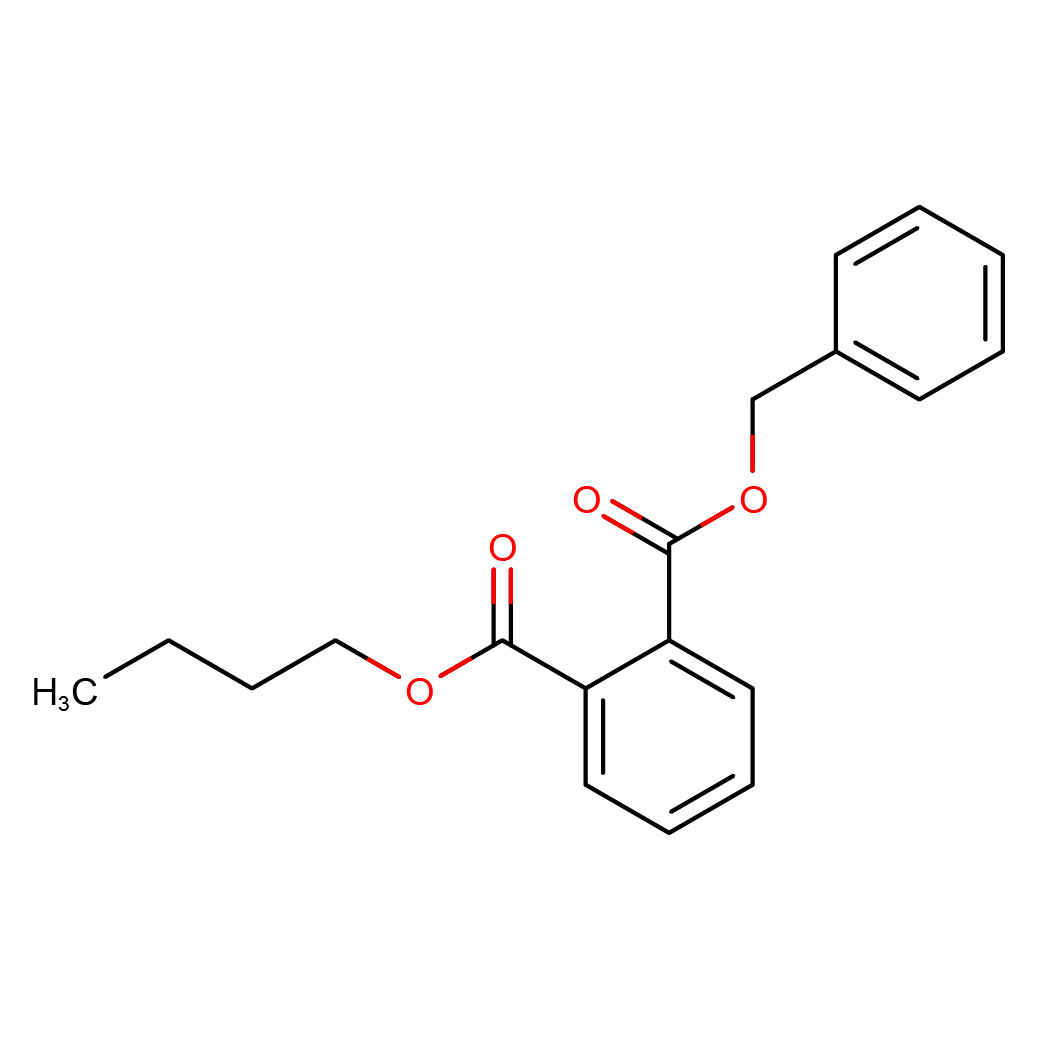
3D-structure