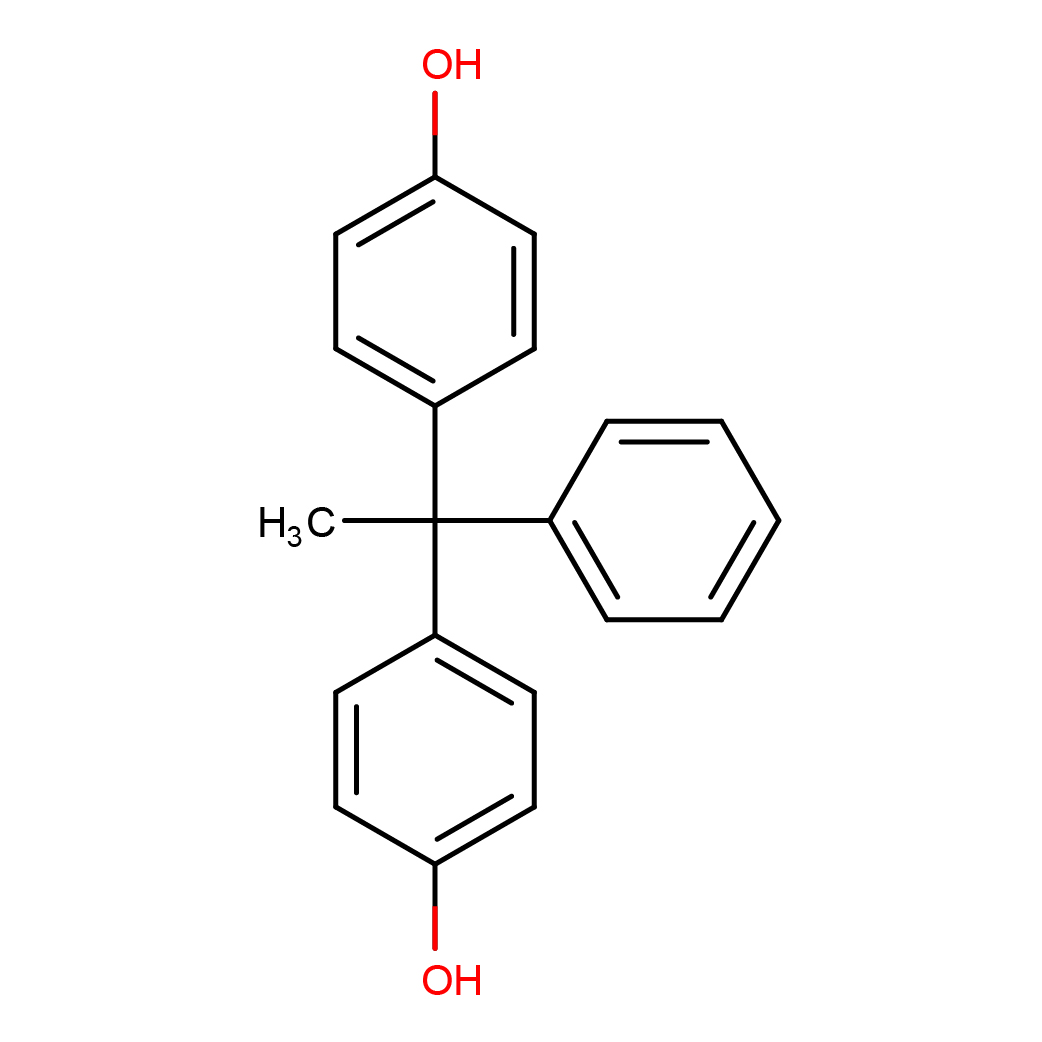
4,4'-(1-phenylethylidene)bisphenol
Synonyms: "4,4'-(1-phenylethane-1,1-diyl)diphenol", "4,4'-(1-Phenylethylidene)bisphenol", "Bisphenol AP", "4-[1-(4-hydroxyphenyl)-1-phenylethyl]phenol", "4,4'-(1-Phenylethylidene) biphenol", "4,4'-(alpha-Methylbenzylidene)bisphenol", "Phenol, 4,4'-(1-phenylethylidene)bis-", "4-[1-(4-hydroxyphenyl)-1-phenyl-ethyl]phenol", "VOWWYDCFAISREI-UHFFFAOYSA-N", "4,4-(1-Phenylethylidene) biphenol", "4,4-(1-phenylethane-1,1-diyl)diphenol", "1,1-Bis(4-hydroxyphenyl)-1-phenylethane".
Source: Bisphenol AP is a plasticizer used as an alternative of bisphenol a.
Identifiers:
IUPAC Name: 4-[1-(4-hydroxyphenyl)-1-phenylethyl]phenol
CAS Number: 1571-75-1
PubChem ID: 623849
InChiKey: VOWWYDCFAISREI-UHFFFAOYSA-N
Canonical SMILES: CC(C1=CC=CC=C1)(C2=CC=C(C=C2)O)C3=CC=C(C=C3)O
Structural Properties:
Molecular Formula: C20H18O2
Molecular Weight: 290.362
Pharmacophore Features:
Number of bond donors: 2
Number of bond acceptors: 2
Number of atoms different from hydrogen: 22
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Coleman KP, Toscano WA, Jr., Wiese TE. 2003. Qsar models of the in vitro estrogen activity of bisphenol a analogs. QSAR Comb Sci 22:78-88 DOI: 10.1002/qsar.200390008. URL: http://onlinelibrary.wiley.com/doi/10.1002/qsar.200390008/abstract.
Kobayashi S, Shinohara H, Tabata K, Yamamoto N and Miyai N. 2006. Stereo structure-controlled and electronic structure-controlled estrogen-like chemicals to design and develop non-estrogenic bisphenol A analogs based on chemical hardness concept. Chem. Pharm. Bull. 54(12):1633-1638. DOI: 10.1248/cpb.54.1633. URL: https://www.jstage.jst.go.jp/article/cpb/54/12/54_12_1633/_articleZhang HC, Hu XL, Yin DQ, Lin ZF. 2011. Development of molecular docking-based binding energy to predict the joint effect of BPA and its analogs. Hum Exp Toxicol 30(4):318-327. DOI: 10.1177/0960327110372400. URL: http://journals.sagepub.com/doi/abs/10.1177/0960327110372400?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed.
External Links

2D-structure
3D-structure