2-aminophenol
Synonyms: "o-aminophenol", "o-hydroxyaniline", "2-hydroxyaniline", "2-amino-1-hydroxybenzene", "Fouramine OP", "Benzofur GG", "Pelagol Grey GG", "Pelagol 3GA", "Nako Yellow 3GA", "ortho-aminophenol", "BASF ursol 3GA", "Zoba 3GA", "Nako Yellow ga", "2-Hydroxyanaline", "Aminophenol", "o-Hydroxyphenylamine", "Paradone Olive Green B", "Questiomycin B", "2-Aminobenzenol", "1-Hydroxy-2-aminobenzene", "1-Amino-2-hydroxybenzene", "2-amino-phenol".
Source: 2-aminophenol is used as the precursor for indols synthesis.
Identifiers:
IUPAC Name: 2-aminophenol
CAS Number: 95-55-6
PubChem ID: 5801
InChiKey: CDAWCLOXVUBKRW-UHFFFAOYSA-N
Canonical SMILES: C1=CC=C(C(=C1)N)O
Structural Properties:
Molecular Formula: C6H7NO
Molecular Weight: 109.128
Pharmacophore Features:
Number of bond donors: 2
Number of bond acceptors: 2
Number of atoms different from hydrogen: 8
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Rogers, K.S. and Evangelista, S.J., 1985. 3-Hydroxykynurenine, 3-hydroxyanthranilic acid, and o-aminophenol inhibit leucine-stimulated insulin release from rat pancreatic islets. Proceedings of the Society for Experimental Biology and Medicine, 178(2), pp.275-278. DOI: https://doi.org/10.3181/00379727-178-42010. URL: http://journals.sagepub.com/doi/abs/10.3181/00379727-178-42010.
External Links

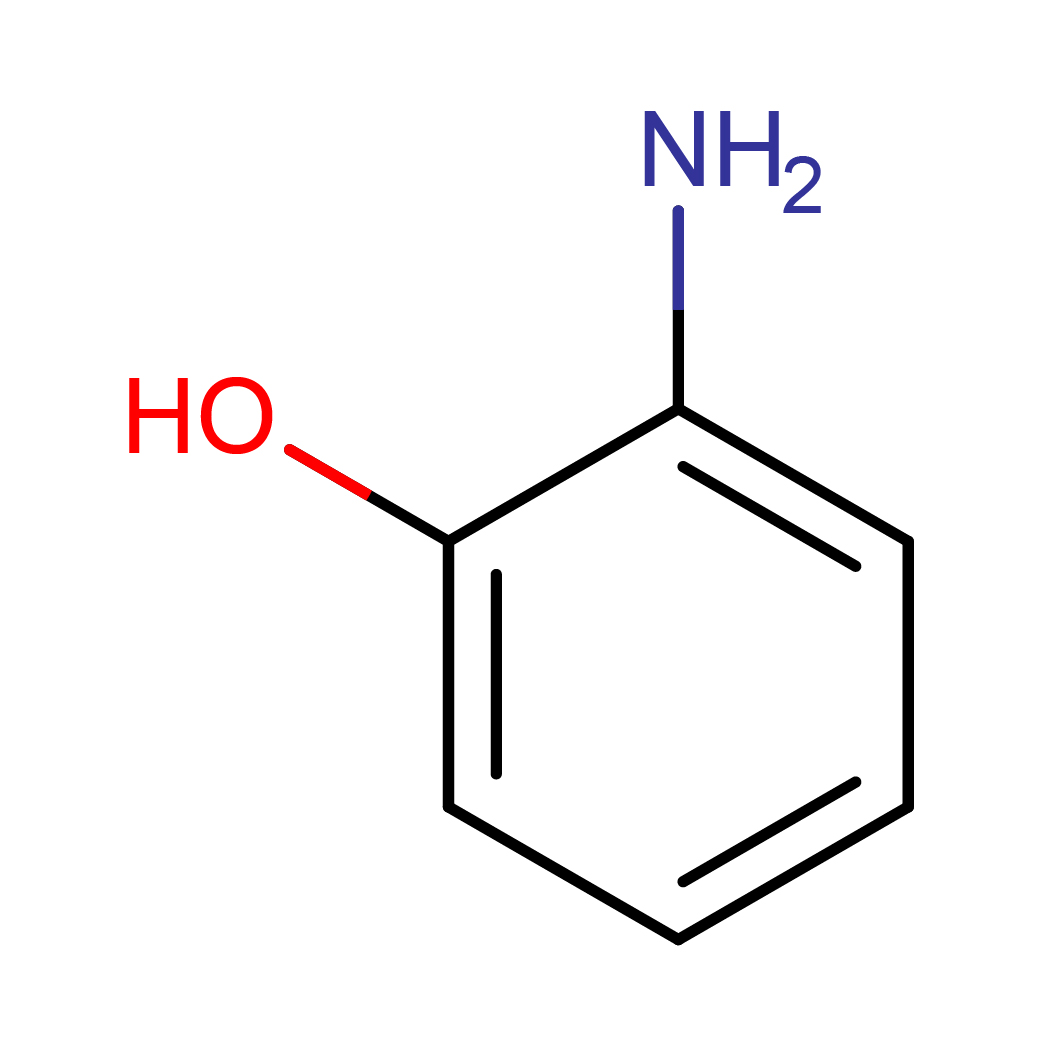
2D-structure
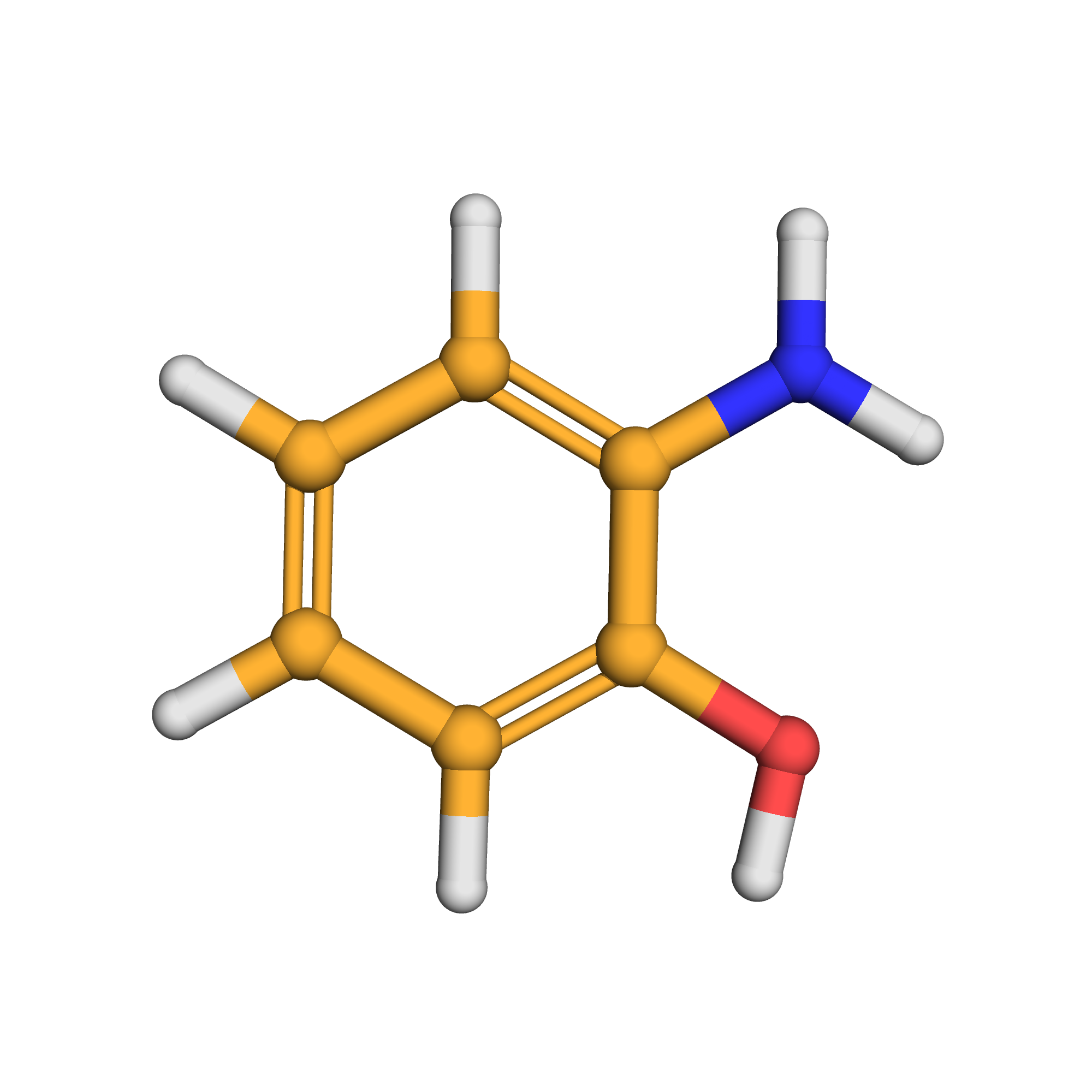
3D-structure