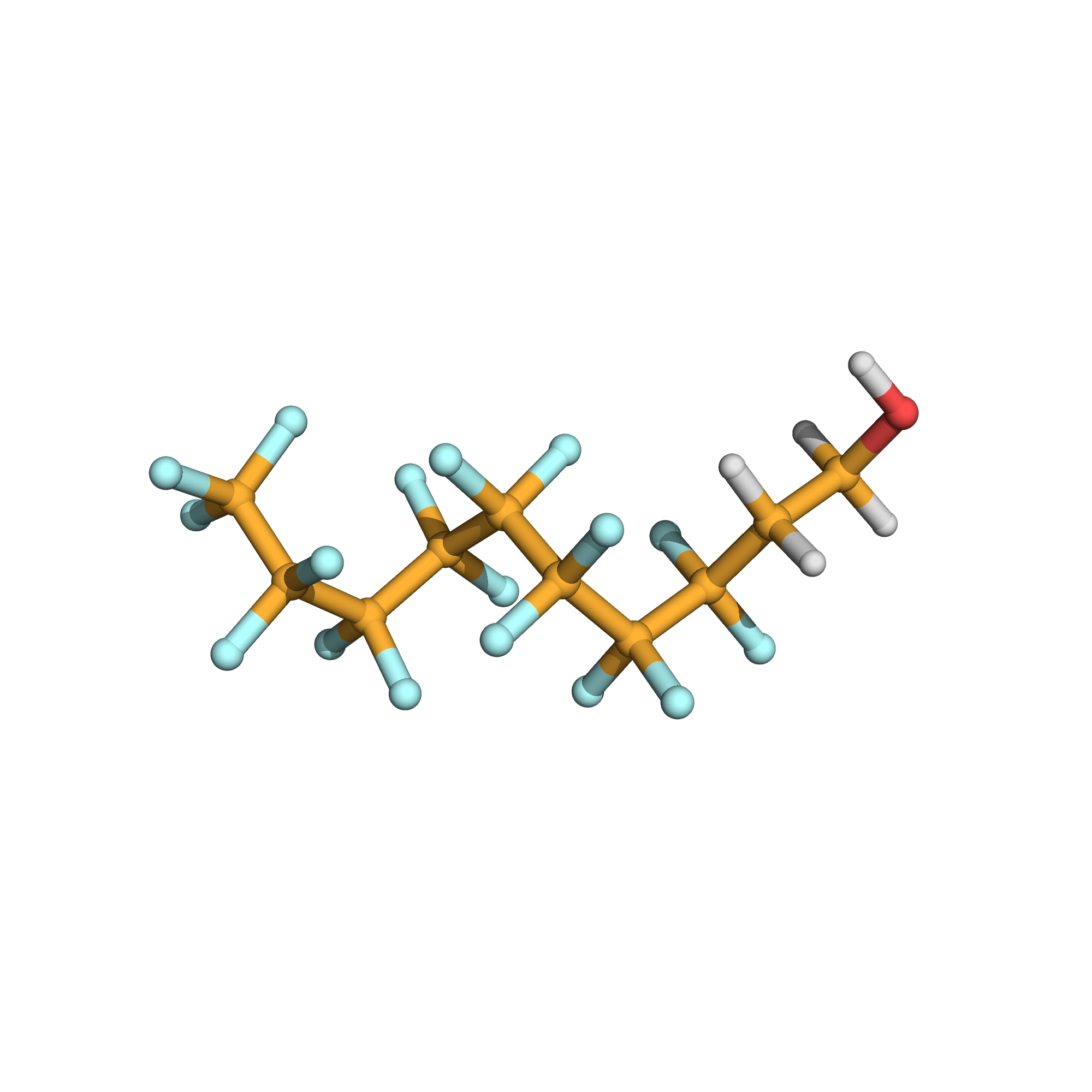
1H,1H,2H,2H-perfluoro-1-decanol
Synonyms: "1H,1H,2H,2H-Perfluoro-1-decanol", "2-(Perfluoro-n-octyl)ethanol", "1H,1H,2H,2H-Perfluorodecanol", "1,1,2,2-Tetrahydroperfluoro-1-decanol", "2-(perfluorooctyl)ethanol", "1H,1H,2H,2H-Perfluorodecan-1-ol", "3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,10-Heptadecafluorodecan-1-ol", "8:2 fluorotelomer alcohol", "8:2 FTOH", "1H,1H,2H,2H-Heptadecafluoro-1-decanol".
Source: 1H,1H,2H,2H-perfluoro-1-decanol, is used as a precursor in the manufacture of perfluorinated carboxylic acids.
Identifiers:
IUPAC Name: 3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,10-heptadecafluorodecan-1-ol
CAS Number: 678-39-7
PubChem ID: 69619
InChiKey: JJUBFBTUBACDHW-UHFFFAOYSA-N
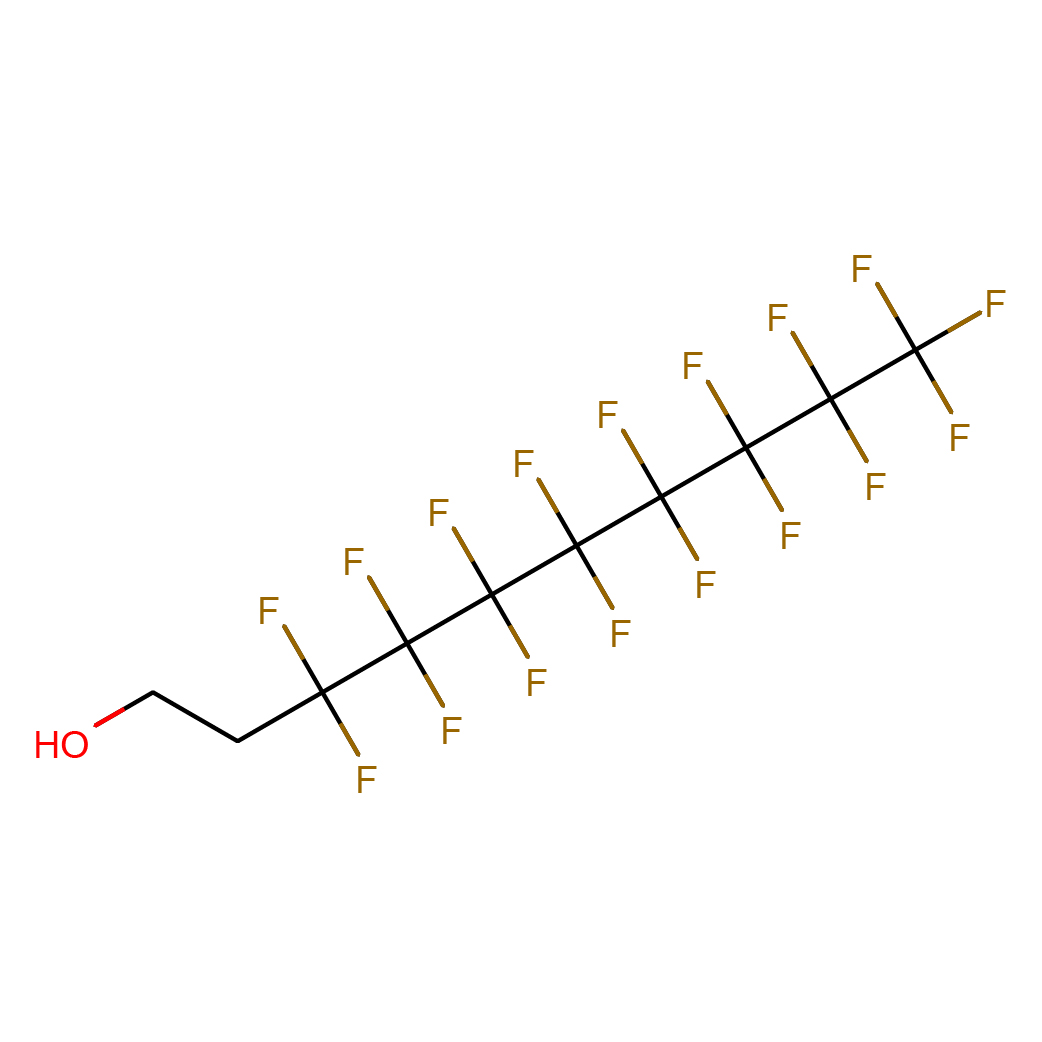
Canonical SMILES: C(CO)C(C(C(C(C(C(C(C(F)(F)F)(F)F)(F)F)(F)F)(F)F)(F)F)(F)F)(F)F
Structural Properties:
Molecular Formula: C10H5F17O
Molecular Weight: 464.122
Pharmacophore Features:
Number of bond donors: 1
Number of bond acceptors: 18
Number of atoms different from hydrogen: 28
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Ishibashi H, Ishida H, Matsuoka M, Tominaga N and Arizono K. 2007. Estrogenic effects of fluorotelomer alcohols for human estrogen receptor isoforms ? and ? in vitro. Biological and Pharmaceutical Bulletin 30(7):1358-1359. DOI: 10.1248/bpb.30.1358. URL: https://www.ncbi.nlm.nih.gov/pubmed/17603182Ishibashi.
H, Yamauchi R, Matsuoka M, Kim JW, Hirano M, Yamaguchi A, Tominaga N and Arizono K. 2008. Fluorotelomer alcohols induce hepatic vitellogenin through activation of the estrogen receptor in male medaka (Oryzias latipes). Chemosphere 71(10):1853-1859. DOI: 10.1016/j.chemosphere.2008.01.065. URL: https://www.ncbi.nlm.nih.gov/pubmed/18334264.
Liu C, Deng J, Yu L, Ramesh M and Zhou B. 2010. Endocrine disruption and reproductive impairment in zebrafish by exposure to 8: 2 fluorotelomer alcohol. Aquatic Toxicology 96(1):70-76. DOI: 10.1016/j.aquatox.2009.09.012. URL: https://www.ncbi.nlm.nih.gov/pubmed/19879662.
Liu C, Du Y and Zhou B. 2007. Evaluation of estrogenic activities and mechanism of action of perfluorinated chemicals determined by vitellogenin induction in primary cultured tilapia hepatocytes. Aquatic toxicology 85(4):267-277. DOI: 10.1016/j.aquatox.2007.09.009. URL: https://www.ncbi.nlm.nih.gov/pubmed/17980923.
Liu C, Zhang X, Chang H, Jones P, Wiseman S, Naile J, Hecker M, Giesy JP and Zhou B. 2010. Effects of fluorotelomer alcohol 8: 2 FTOH on steroidogenesis in H295R cells: targeting the cAMP signalling cascade. Toxicol Appl Pharmacol 247(3):222-228. DOI: 10.1016/j.taap.2010.06.016. URL: https://www.ncbi.nlm.nih.gov/pubmed/20615422.
Maras M, Vanparys C, Muylle F, Robbens J, Berger U, Barber JL, Blust R and De Coen W. 2006. Estrogen-like properties of fluorotelomer alcohols as revealed by MCF-7 breast cancer cell proliferation. Environ Health Perspect 114(1):100-5. DOI: 10.1289/ehp.8149. URL: https://www.ncbi.nlm.nih.gov/pubmed/16393665.
Rosenmai AK, Nielsen FK, Pedersen M, Hadrup N, Trier X, Christensen JH, Vinggaard AM. 2013 Jan 1. Fluorochemicals used in food packaging inhibit male sex hormone synthesis. Toxicol Appl Pharmacol 266(1):132-142. DOI: 10.1016/j.taap.2012.10.022. URL: https://www.sciencedirect.com/science/article/pii/S0041008X12004644?via%3Dihub.
Rosenmai AK, Nielsen FK, Pedersen M, Hadrup N, Trier X, Christensen JH, Vinggaard AM. 2013 Jan 1. Fluorochemicals used in food packaging inhibit male sex hormone synthesis. Toxicol Appl Pharmacol 266(1):132-142. DOI: 10.1016/j.taap.2012.10.022. URL: https://www.sciencedirect.com/science/article/pii/S0041008X12004644?via%3Dihub
External Links

2D-structure
3D-structure