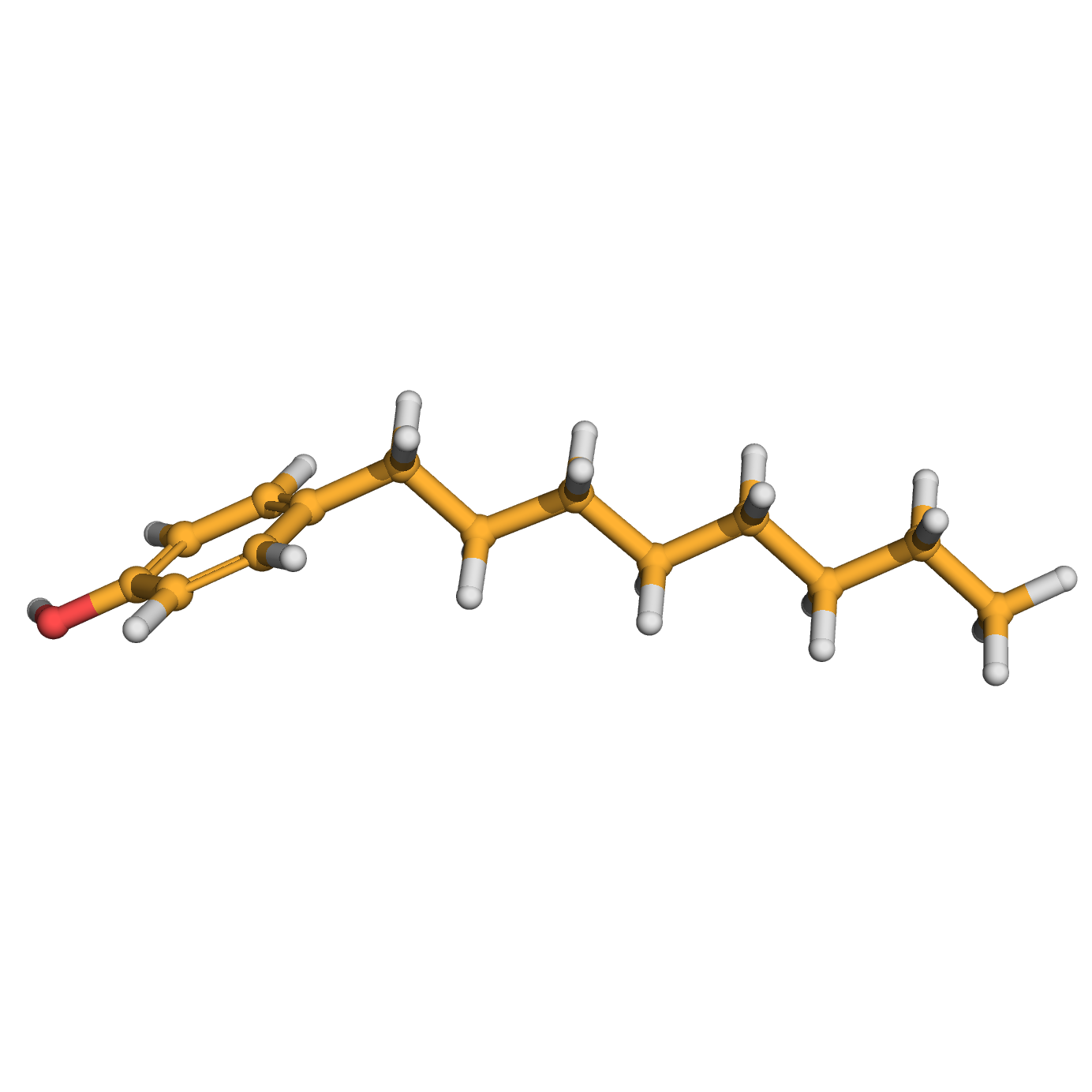
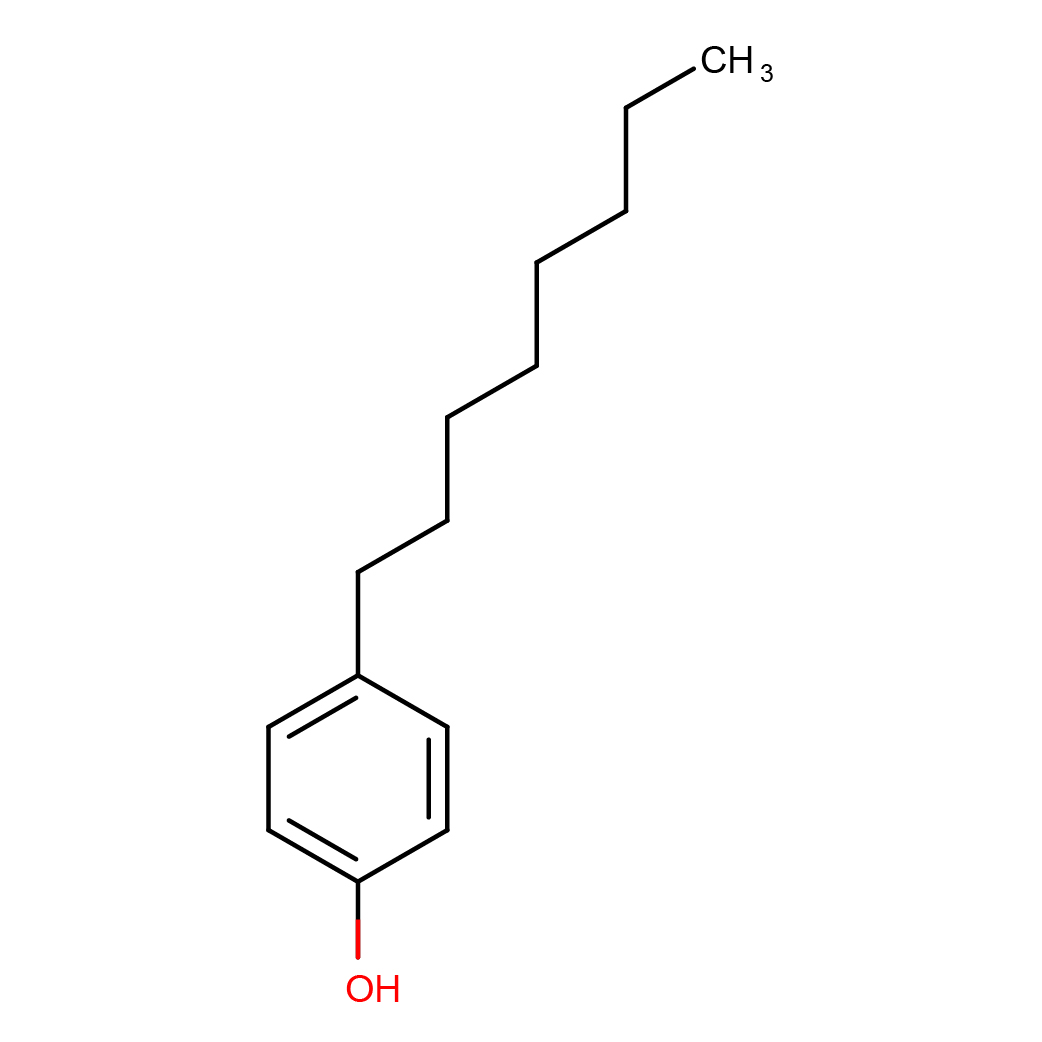
4-n-octylphenol
Synonyms: "4-octylphenol", "p-octylphenol", "4-n-octylphenol", "para-octylphenol", "1-(p-hydroxyphenyl)octane"
Source: 4-n-octylphenol is used in the manufacture of surfactants, plasticizers and other chemicals.
Identifiers:
IUPAC Name: 4-octylphenol
CAS Number: 1806-26-4
PubChem ID: 15730
InChiKey: NTDQQZYCCIDJRK-UHFFFAOYSA-N
Canonical SMILES: CCCCCCCCC1=CC=C(C=C1)O
Structural Properties:
Molecular Formula: C14H22O
Molecular Weight: 206.324
Pharmacophore Features:
Number of bond donors: 1
Number of bond acceptors: 1
Number of atoms different from hydrogen: 15
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Kanayama T, Kobayashi N, Mamiya S, Nakanishi T, Nishikawa J. 2005. Organotin compounds promote adipocyte differentiation as agonists of the peroxisome proliferator-activated receptor [gamma]/retinoid X receptor pathway. Mol Pharmacol 67(3):766-774.
Nakagomi M, Suzuki E, Usumi K, Saitoh Y, Yoshimura S, Nagao T, Ono H. 2001. Effects of endocrine disrupting chemicals on the microtubule network in Chinese hamster V79 cells in culture and in Sertoli cells in rats. Teratogenesis, Carcinogenesis & Mutagenesis 21(6):453-462.
Schultz TW, Sinks GD, Cronin MTD. 2000. Effect of substituent size and dimensionality on potency of phenolic xenoestrogens evaluated with a recombinant yeast assay. Environ Toxicol Chem 19(11):2637-2642.
Soto AM, Sonnenschein C, Chung KL, Fernandez MF, Olea N, Serrano FO. 1995. The E-SCREEN assay as a tool to identify estrogens: An update on estrogenic environmental pollutants. Environ Health Perspect 103 (Suppl. 7):113-122.
External Links

2D-structure
3D-structure