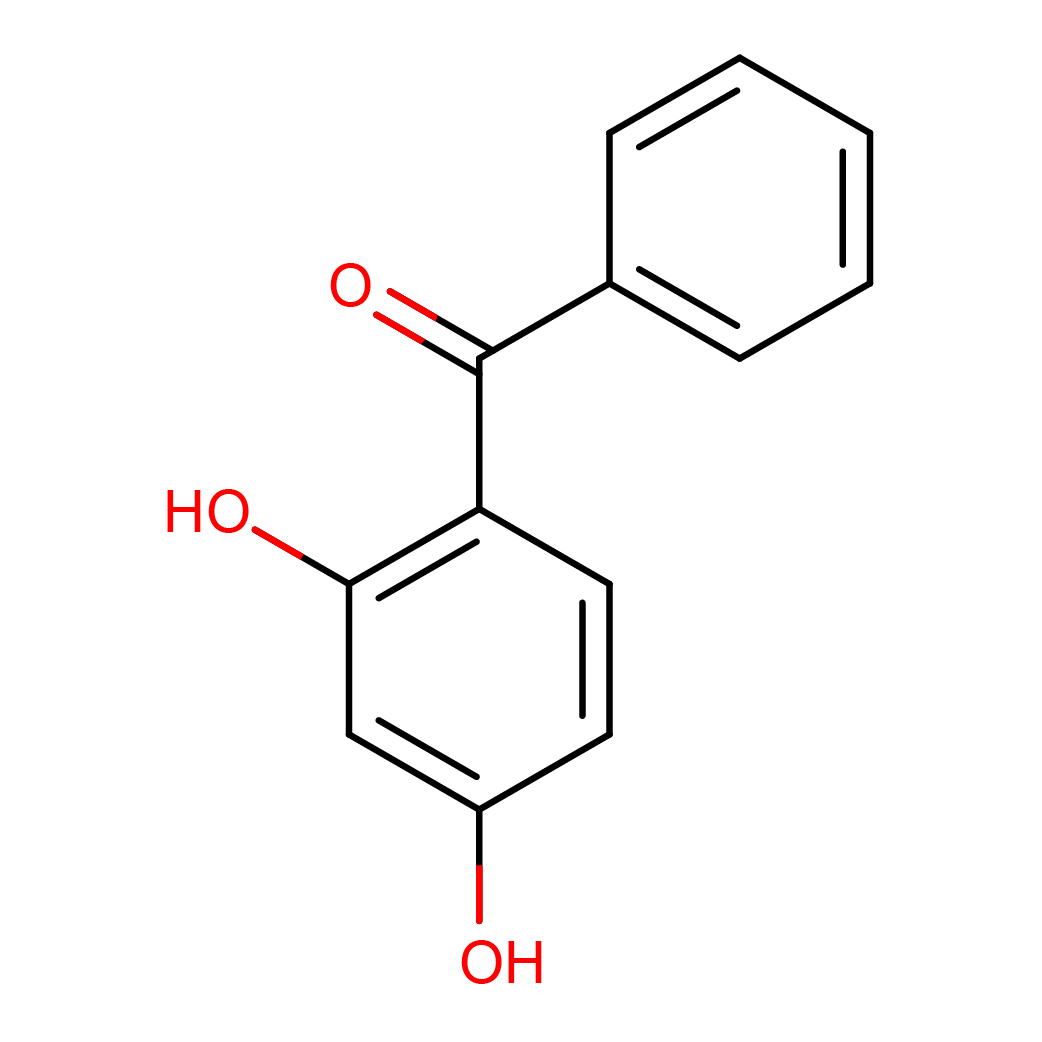
2,4-dihydroxybenzophenone
Synonyms: "benzoresorcinol", "benzophenone-1", "resbenzophenone", "inhibitor DHBP", "advastab 48", "uvistat 12", "4-benzoyl resorcinol", "uvinol 400"
Source: 2,4-dihydroxybenzophenone is used as a constituent of synthetic perfumes and as a starting material for the manufacture of dyes, pesticides and drugs (especially anxiolytic and hypnotic drugs). It is used as a photoinitiator of UV-curing applications in inks, adhesive and coatings,optical fiber.
Identifiers:
IUPAC Name: (2,4-dihydroxyphenyl)-phenylmethanone
CAS Number: 131-56-6
PubChem ID: 8572
InChiKey: ZXDDPOHVAMWLBH-UHFFFAOYSA-N
Canonical SMILES: C1=CC=C(C=C1)C(=O)C2=C(C=C(C=C2)O)O
Structural Properties:
Molecular Formula: C13H10O3
Molecular Weight: 214.217
Pharmacophore Features:
Number of bond donors: 2
Number of bond acceptors: 3
Number of atoms different from hydrogen: 16
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Molina-Molina JM, Escande A, Pillon A, Gomez E, Pakdel F, Cavailles V, Olea N, Ait-Aissa S, Balaguer P. 2008. Profiling of benzophenone derivatives using fish and human estrogen receptor-specific in vitro bioassays. Toxicol Appl Pharmacol 232(3):384-395.
Nakagawa Y, Suzuki T. 2002. Metabolism of 2-hydroxy-4-methoxybenzophenone in isolated rat hepatocytes and xenoestrogenic effects of its metabolites on MCF-7 human breast cancer cells. Chem Biol Interact 139(2):115-128.
Schlumpf M, Schmid P, Durrer S, Conscience M, Maerkel K, Henseler M, Gruetter M, Herzog I, Reolon S, Ceccatelli R, Faass O, Stutz E, Jarry H, Wuttke W, Lichtensteiger W. 2004. Endocrine activity and developmental toxicity of cosmetic UV filters - an update. Toxicology 205(1-2):113-122.
Suzuki T, Kitamura S, Khota R, Sugihara K, Fujimoto N, Ohta S. 2005. Estrogenic and antiandrogenic activities of 17 benzophenone derivatives used as UV stabilizers and sunscreens. Toxicol Appl Pharmacol 203(1):9-17.Yamasaki K, Noda S, Imatanaka N, Yakabe Y. 2004. Comparative study of the uterotrophic potency of 14 chemicals in a uterotrophic assay and their receptor-binding affinity. Toxicol Lett 146(2):111-120.
External Links




2D-structure
3D-structure