epoxyheptachlor
Synonyms: "cis-heptachlor epoxide", "(+/-)-cis-heptachlorepoxide", "heptepoxide", "hepox", "2,3,4,5,6,7,7-Heptachloro-1a,1b,5,5a,6,6a-hexahydro-2,5-methano-2H-indeno(1,2-b)oxirene", "1,4,5,6,7,8,8-Heptachloro-2,3-epoxy-3a,4,7,7a-tetrahydro-4,7-methanoindan".
Source: epoxyheptachlor is an oxidation product of heptachlor formed by many plants and animals, including humans, after exposure to this compound.
Identifiers:
IUPAC Name: (1S,2R,3S,5S,6R,7S,8R)-1,6,8,9,10,11,11-heptachloro-4-oxatetracyclo[6.2.1.02,7.03,5]undec-9-ene
CAS Number: 1024-57-3
PubChem ID: 15559699
InChiKey: ZXFXBSWRVIQKOD-UOFFAGTMSA-N
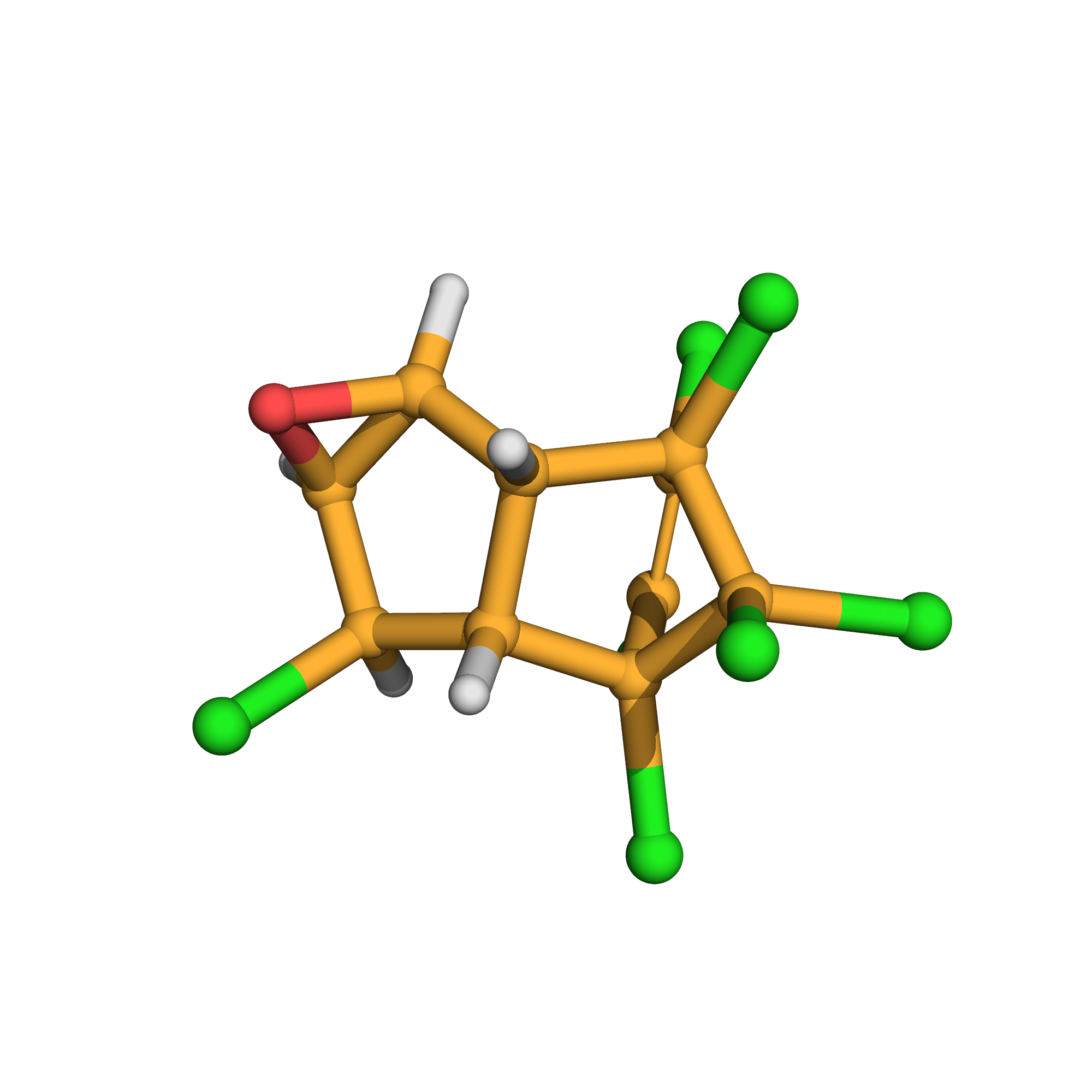
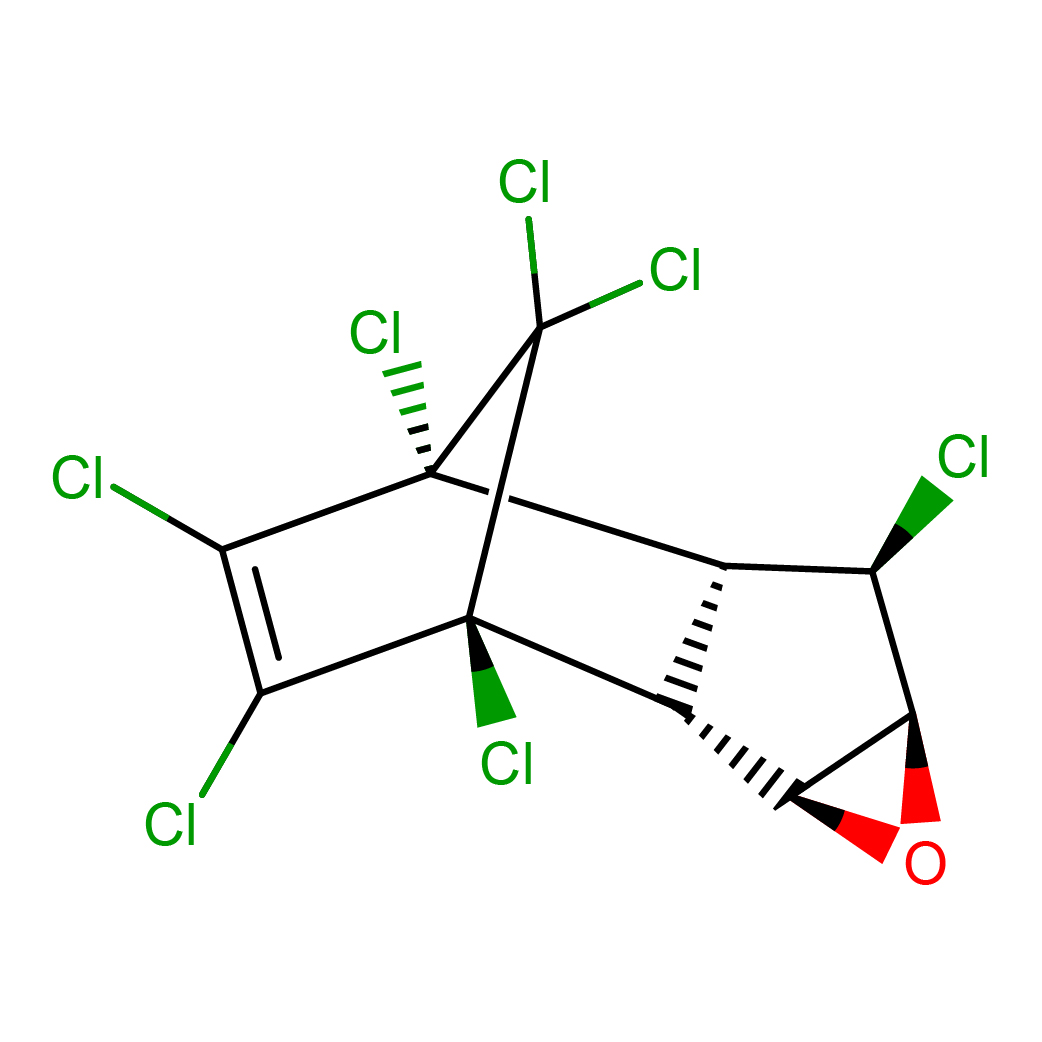
Canonical SMILES: C12C(C(C3C1O3)Cl)C4(C(=C(C2(C4(Cl)Cl)Cl)Cl)Cl)Cl
Structural Properties:
Molecular Formula: C10H5Cl7O
Molecular Weight: 389.299
Pharmacophore Features:
Number of bond donors: 0
Number of bond acceptors: 1
Number of atoms different from hydrogen: 18
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Abalis IM, Eldefrawi ME, Eldefrawi AT. 1985. High-affinity stereospecific binding of cyclodiene insecticides and gamma-hexachlorocyclohexane to gamma-aminobutyric acid receptors of rat brain. Pesticide Biochemistry & Physiology 24(1):95-102. DOI: 10.1016/0048-3575(85)90118-X. URL: https://www.sciencedirect.com/science/article/pii/004835758590118X.
Kojima H, Katsura E, Takeuchi S, Niiyama K, Kobayashi K. 2004. Screening for estrogen and androgen receptor activities in 200 pesticides by in vitro reporter gene assays using Chinese hamster ovary cells. Environ Health Perspect 112(5):524-531. URL: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1241915/.
External Links

2D-structure
3D-structure