dibenzo[a,h]pyrene
Synonyms: "dibenzo[b,def]chrysene", "dibenzo[a,h]pyrene", "dibenzo(a,h)pyrene", "DB(a,h)P", "3,4,8,9-dibenzopyrene", "1,2,6,7-dibenzopyrene", "dibenz[a,h]pyrene", "dibenzo(a,h)pyrene", "1,2,6,7-Dibenzpyrene".
Source: dibenzo[a,h]pyrene is a dibenzopyrene isomer. Primary sources of dibenzopyrenes in the environment are combustion of wood and coal, gasoline and diesel exhaust, and tires.
Identifiers:
IUPAC Name: hexacyclo[10.10.2.02,7.09,23.013,18.020,24]tetracosa-1(23),2,4,6,8,10,12(24),13,15,17,19,21-dodecaene
CAS Number: 189-64-0
PubChem ID: 9108
InChiKey: RXUSYFJGDZFVND-UHFFFAOYSA-N
Canonical SMILES: C1=CC=C2C3=C4C(=CC2=C1)C=CC5=C4C(=CC6=CC=CC=C56)C=C3
Structural Properties:
Molecular Formula: C24H14
Molecular Weight: 302.376
Pharmacophore Features:
Number of bond donors: 0
Number of bond acceptors: 0
Number of atoms different from hydrogen: 24
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Machala M, Vondracek J, Blaha L, Ciganek M, Neca JV. 2001. Aryl hydrocarbon receptor-mediated activity of mutagenic polycyclic aromatic hydrocarbons determined using in vitro reporter gene assay. Mutat Res 497(1-2):49-62. DOI: 10.1016/S1383-5718(01)00240-6. URL: https://www.sciencedirect.com/science/article/pii/S1383571801002406.
External Links

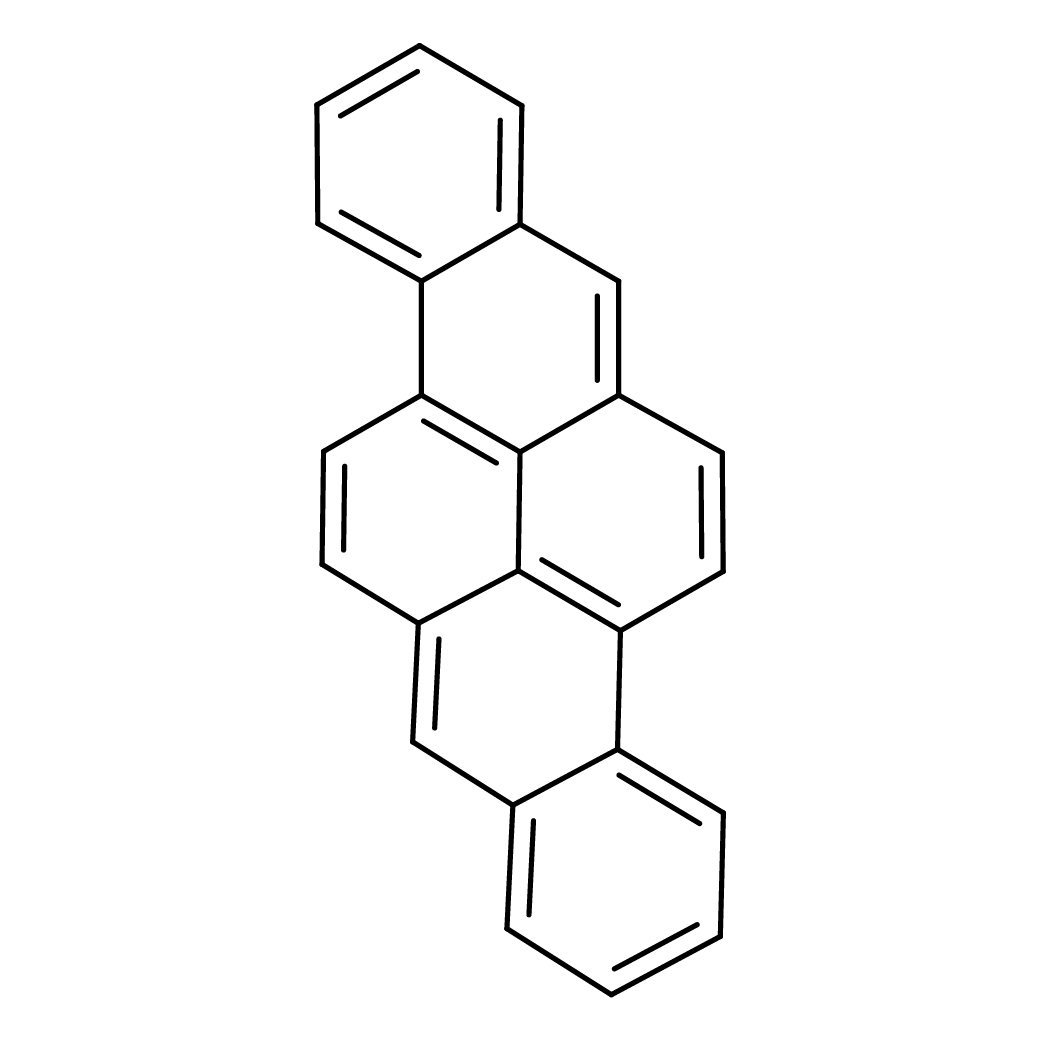
2D-structure
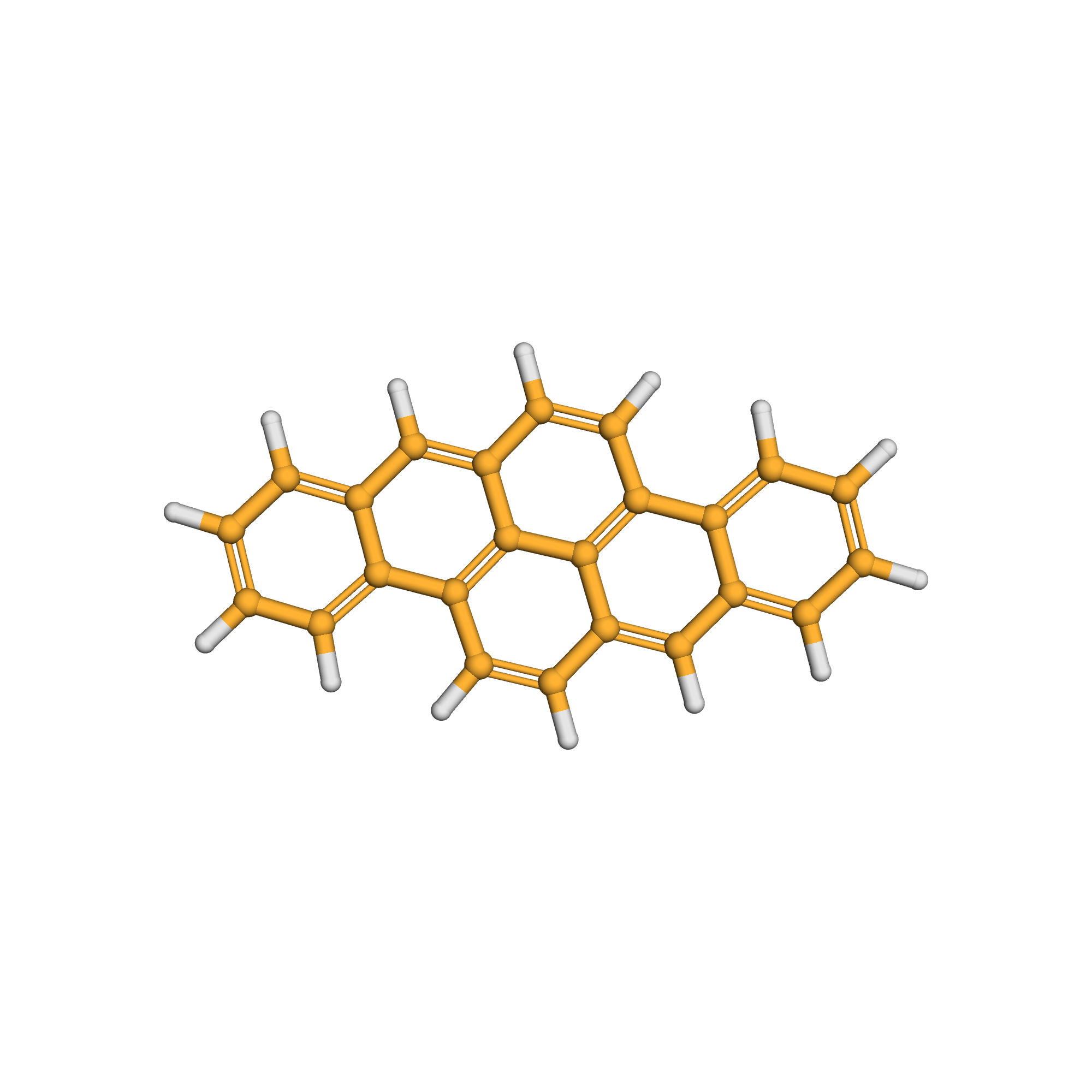
3D-structure