crotonaldehyde
Synonyms: "2-butenal", "crotylaldehyde", "crotonal", "(2E)-but-2-enal", "trans-crotonaldehyde", "(E)-crotonaldehyde", "crotonic aldehyde", "(E)-but-2-enal", "trans-2-butenal", "methylpropenal", "propylene aldehyde", "2-butenal, (2E)-", "1-formylpropene", "beta-methylacrolein", "aldehyde crotonique", "2-butenaldehyde", "but-2-enal", "topanel", "topanel CA", "trans-but-2-enal", "E-2-butenal", "beta-methyl acrolein", "trans-2-butenaldehyde".
Source: Crotonaldehyde is used in large amounts in production of organic compounds, such as sorbic acid and n-butanol.
Identifiers:
IUPAC Name: (E)-but-2-enal
CAS Number: 123-73-9
PubChem ID: 447466
InChiKey: MLUCVPSAIODCQM-NSCUHMNNSA-N
Canonical SMILES: CC=CC=O
Structural Properties:
Molecular Formula: C4H6O
Molecular Weight: 70.091
Pharmacophore Features:
Number of bond donors: 0
Number of bond acceptors: 1
Number of atoms different from hydrogen: 5
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Grundfest CC, Chang J, Newcombe D. 1982. Acrolein: a potent modulator of lung macrophage arachidonic acid metabolism. Biochim Biophys Acta 713(1):149-159. DOI: 10.1016/0005-2760(82)90177-1. URL: https://www.sciencedirect.com/science/article/pii/0005276082901771Jha AM, Kumar M. 2006. In vivo evaluation of induction of abnormal sperm morphology in mice by an unsaturated aldehyde crotonaldehyde. Mutat Res 603(2):159-163. DOI: 10.1016/j.mrgentox.2005.11.010. URL: https://www.sciencedirect.com/science/article/pii/S1383571805002962?via%3Dihub.
External Links

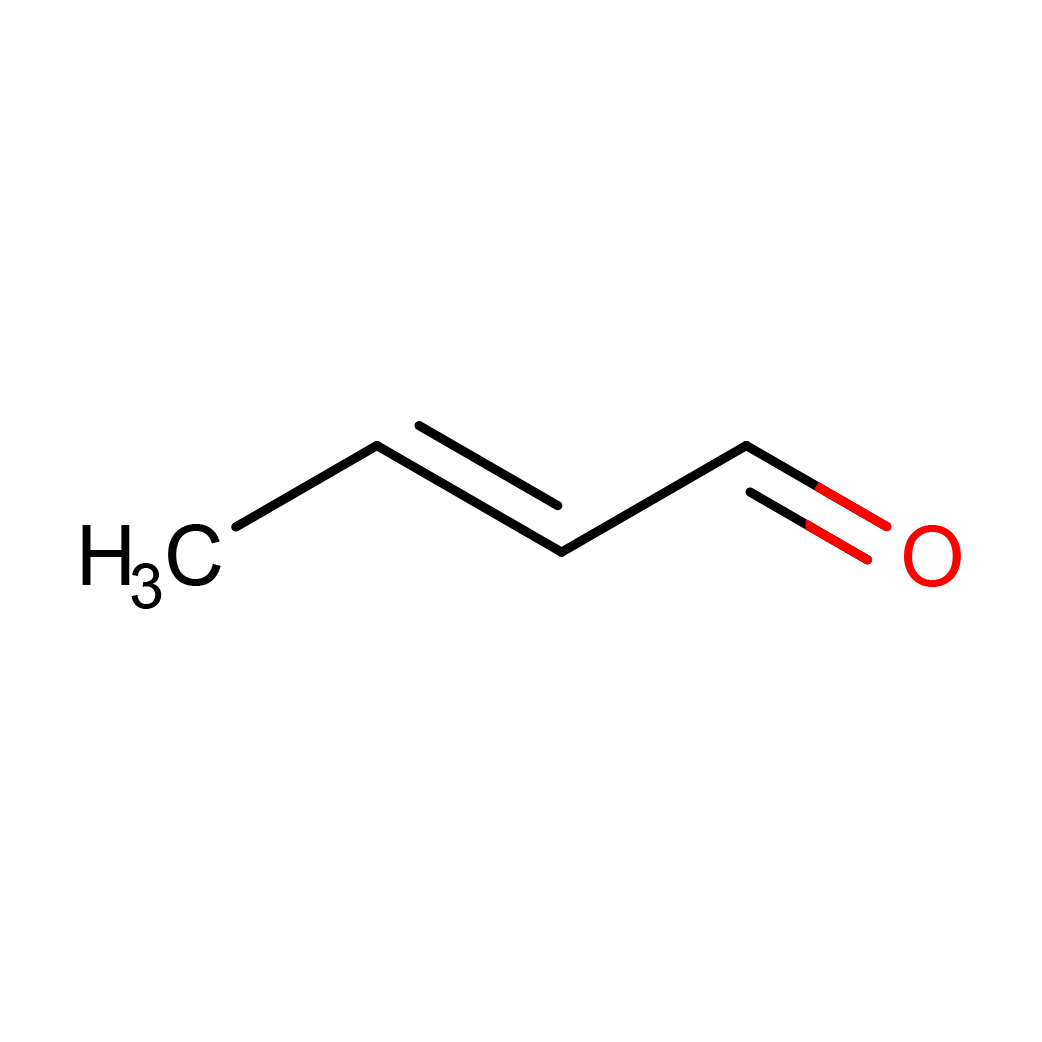
2D-structure
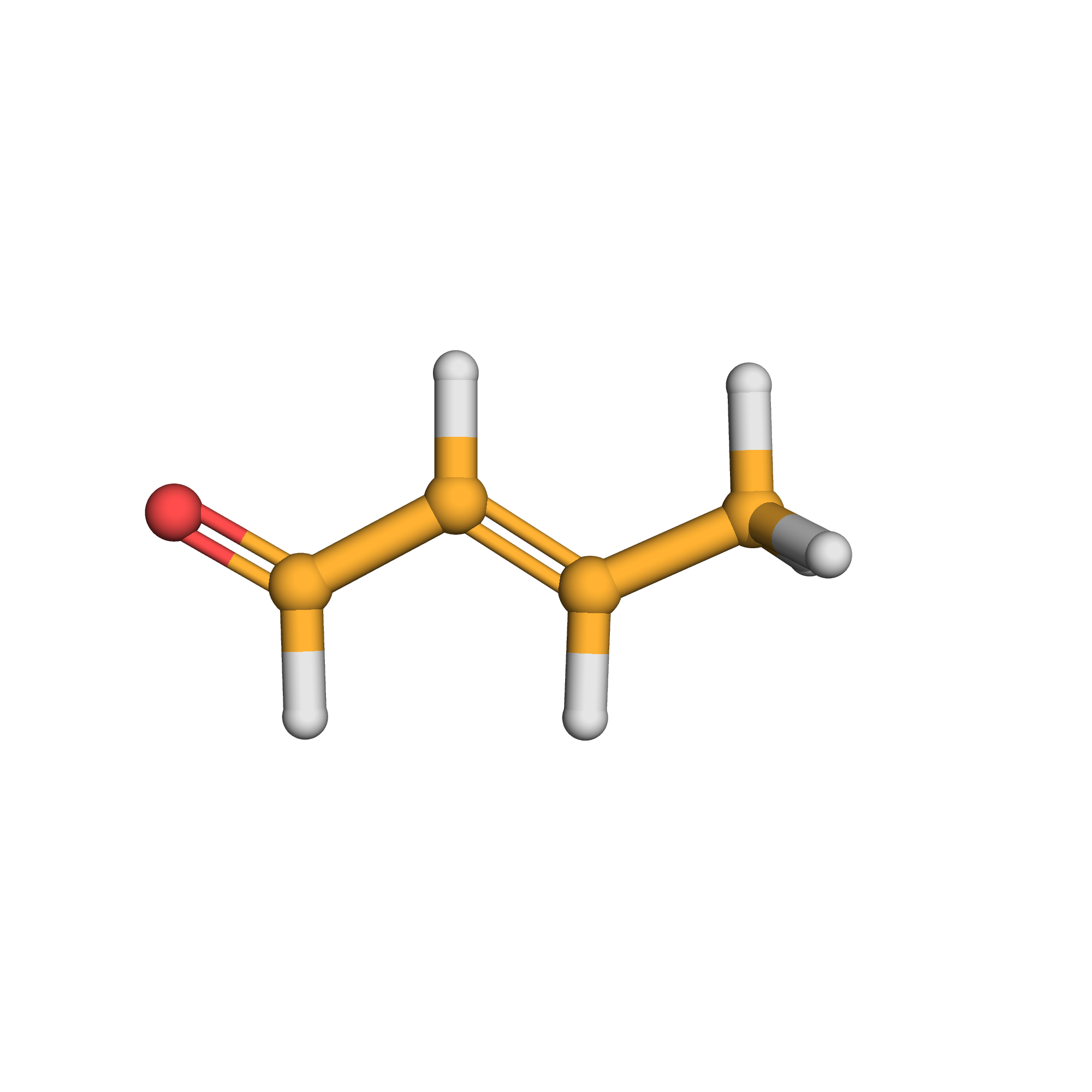
3D-structure