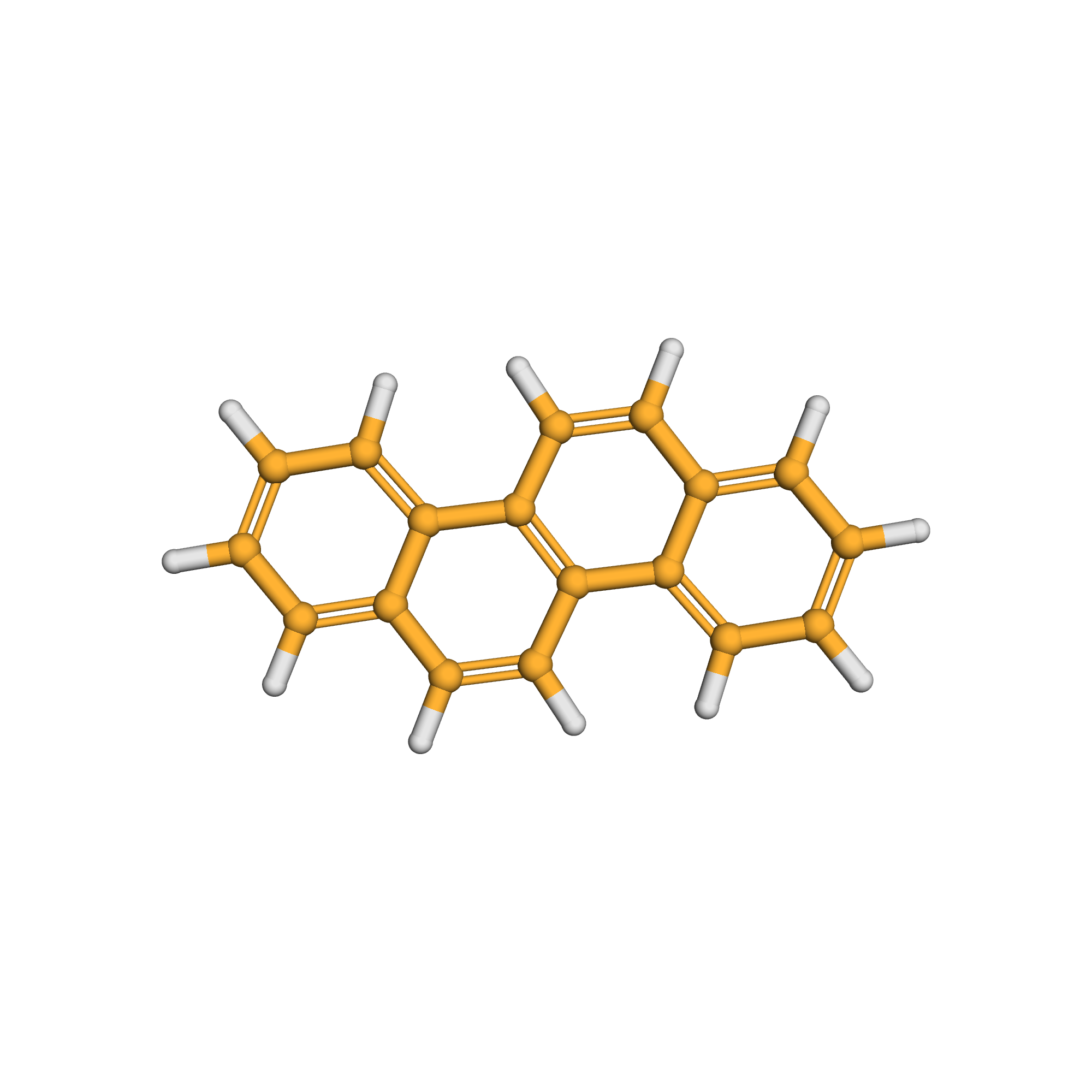
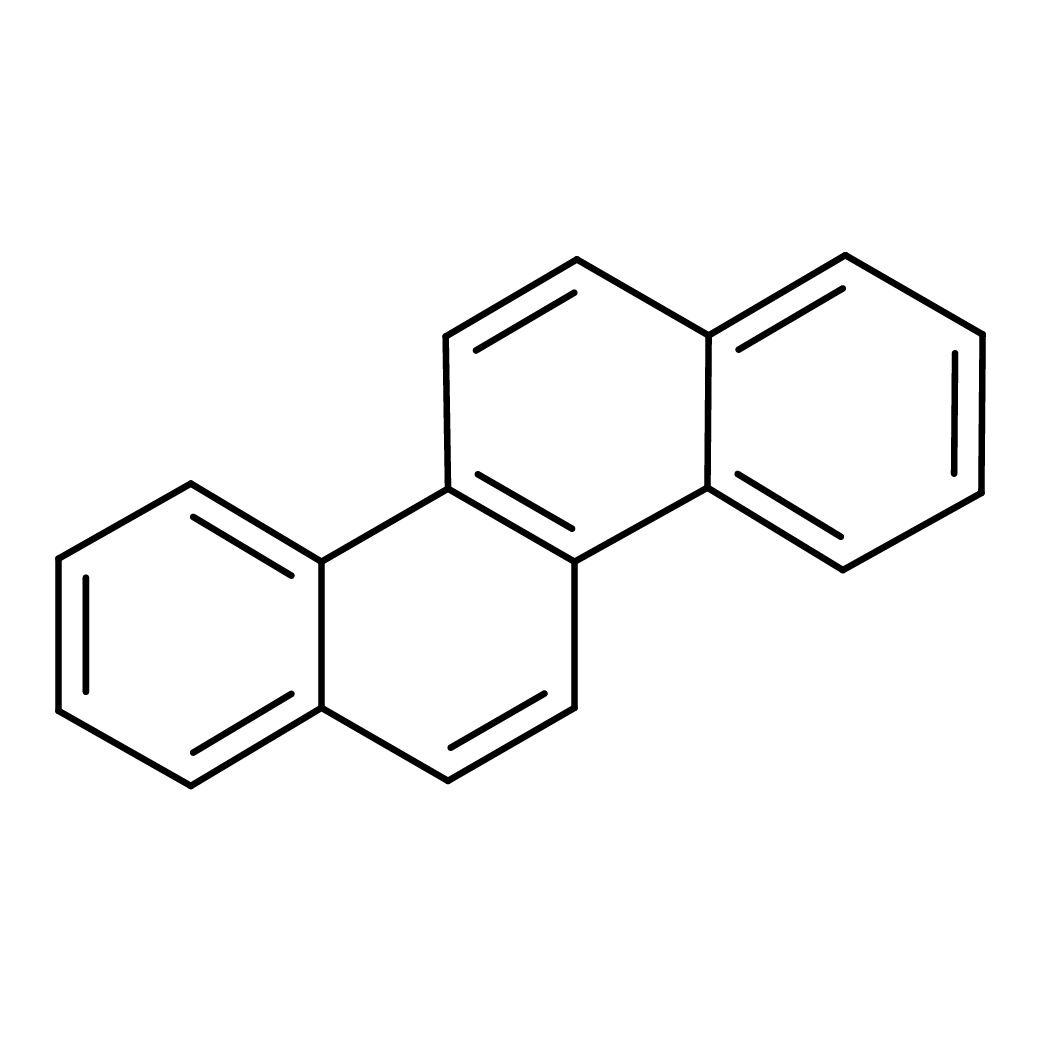
chrysene
Synonyms: "chrysene", "benzo[a]phenanthrene", "1,2-benzophenanthrene", "1,2-benzphenanthrene", "1,2,5,6-dibenzonaphthalene", "benz[a]phenanthrene", "benz(a)phenanthrene", "benzo(a)phenanthrene", "chrysen", "Benzo(a)phenanthrene".
Source: Chrysene is a high molecular weight polycyclic aromatic hydrocarbon, produced during incomplete combustion of organic materials.
Identifiers:
IUPAC Name: chrysene
CAS Number: 218-01-9
PubChem ID: 9171
InChiKey: WDECIBYCCFPHNR-UHFFFAOYSA-N
Canonical SMILES: C1=CC=C2C(=C1)C=CC3=C2C=CC4=CC=CC=C43
Structural Properties:
Molecular Formula: C18H12
Molecular Weight: 228.294
Pharmacophore Features:
Number of bond donors: 0
Number of bond acceptors: 0
Number of atoms different from hydrogen: 18
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Machala M, Vondracek J, Blaha L, Ciganek M, Neca JV. 2001. Aryl hydrocarbon receptor-mediated activity of mutagenic polycyclic aromatic hydrocarbons determined using in vitro reporter gene assay. Mutat Res 497(1-2):49-62. DOI: 10.1016/S1383-5718(01)00240-6. URL: https://www.sciencedirect.com/science/article/pii/S1383571801002406.
Vinggaard AM, Hnida C, Larsen JC. 2000. Environmental polycyclic aromatic hydrocarbons affect androgen receptor activation in vitro. Toxicology 145(2-3):173-183. DOI: 10.1016/S0300-483X(00)00143-8. URL: https://www.sciencedirect.com/science/article/pii/S0300483X00001438.
External Links

2D-structure
3D-structure