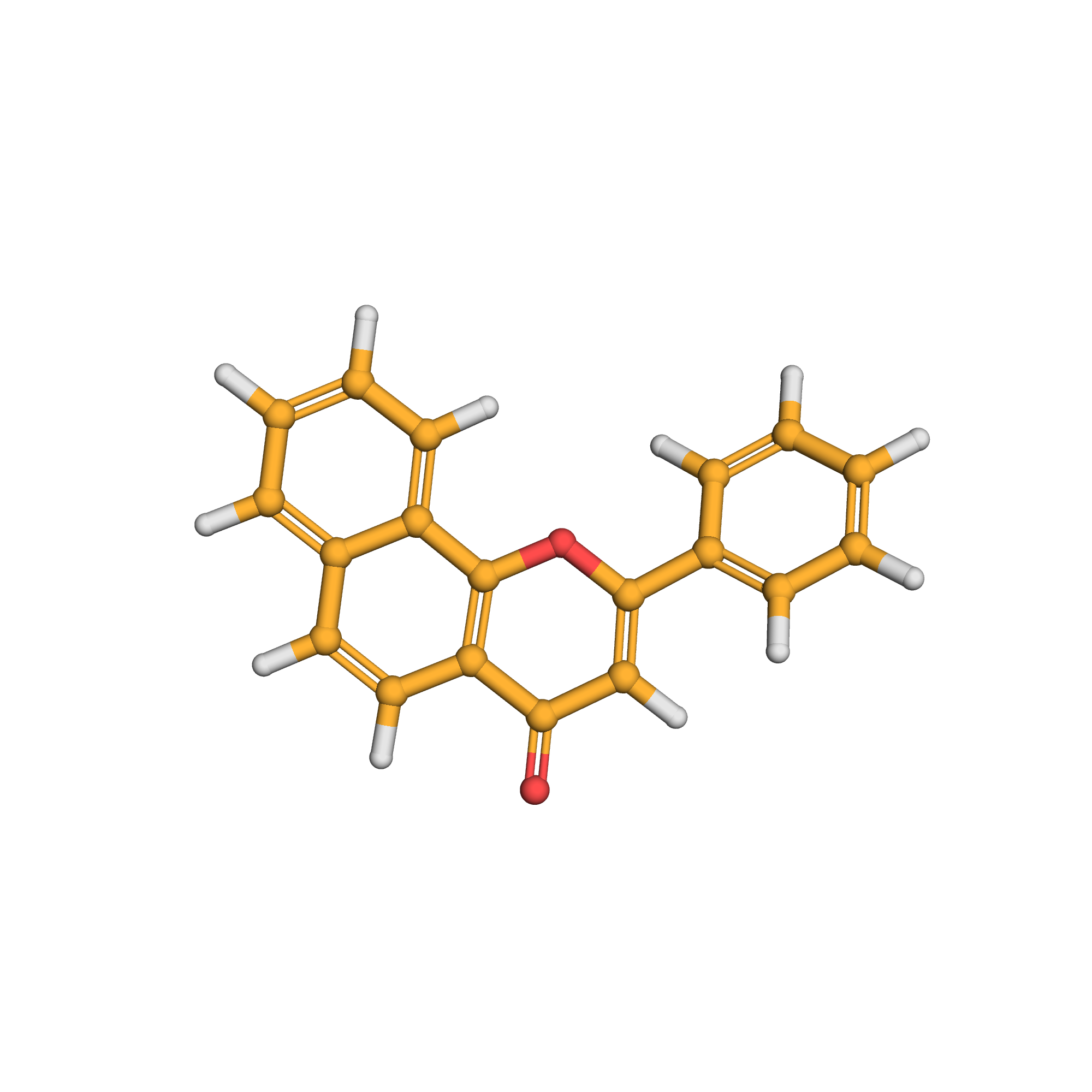
alpha-naphthoflavone
Synonyms: "7,8-benzoflavone", "alpha-naphthoflavone", "2-phenyl-4H-benzo[h]chromen-4-one", "alpha-naphthylflavone", "2-phenylbenzo[h]chromen-4-one", "benzo(h)flavone", "7,8-BF", "2-phenyl-benzo[h]chromen-4-one", "2-phenyl-4H-naphtho(1,2-b)pyran-4-one", "2-phenylbenzo(h)chromen-4-one", "benzo[h]flavone".
Source: alpha-naphthoflavone is a flavonoid.
Identifiers:
IUPAC Name: 2-phenylbenzo[h]chromen-4-one
CAS Number: 604-59-1
PubChem ID: 11790
InChiKey: VFMMPHCGEFXGIP-UHFFFAOYSA-N
Canonical SMILES: C1=CC=C(C=C1)C2=CC(=O)C3=C(O2)C4=CC=CC=C4C=C3
Structural Properties:
Molecular Formula: C19H12O2
Molecular Weight: 272.303
Pharmacophore Features:
Number of bond donors: 0
Number of bond acceptors: 2
Number of atoms different from hydrogen: 21
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Campbell DR, Kurzer MS. 1993. Flavonoid inhibition of aromatase enzyme activity in human preadipocytes. J Steroid Biochem Mol Biol 46(3):381-388. DOI: 10.1016/0960-0760(93)90228-O. URL: https://www.sciencedirect.com/science/article/pii/096007609390228OPelissero C, Lenczowski MJP, Chinzi D, Davail-Cuisset B, Sumpter JP, Fostier A. 1996. Effects of flavonoids on aromatase activity, an in vitro study. J Steroid Biochem Mol Biol 57(3-4):215-223. DOI: 10.1016/0960-0760(95)00261-8. URL: https://www.sciencedirect.com/science/article/pii/0960076095002618.
External Links


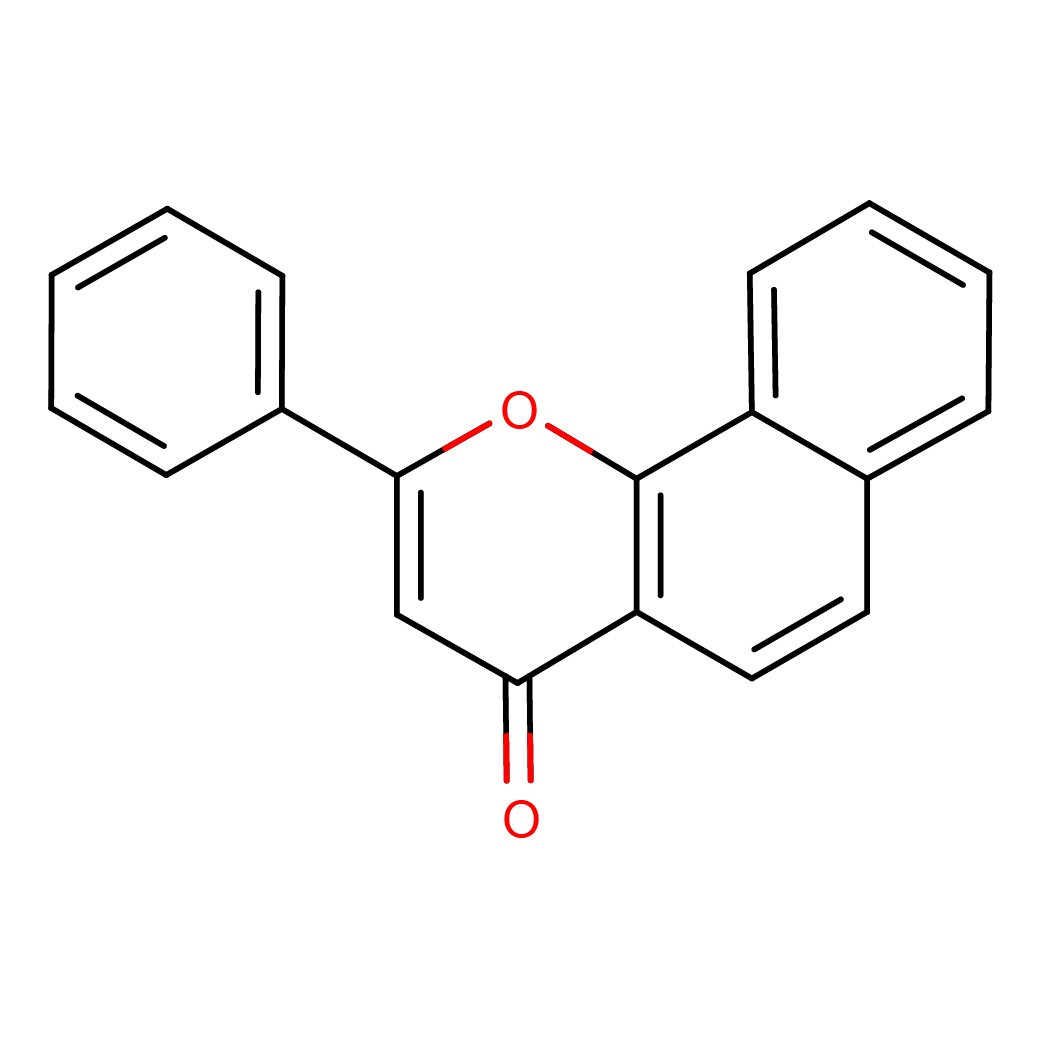
2D-structure
3D-structure