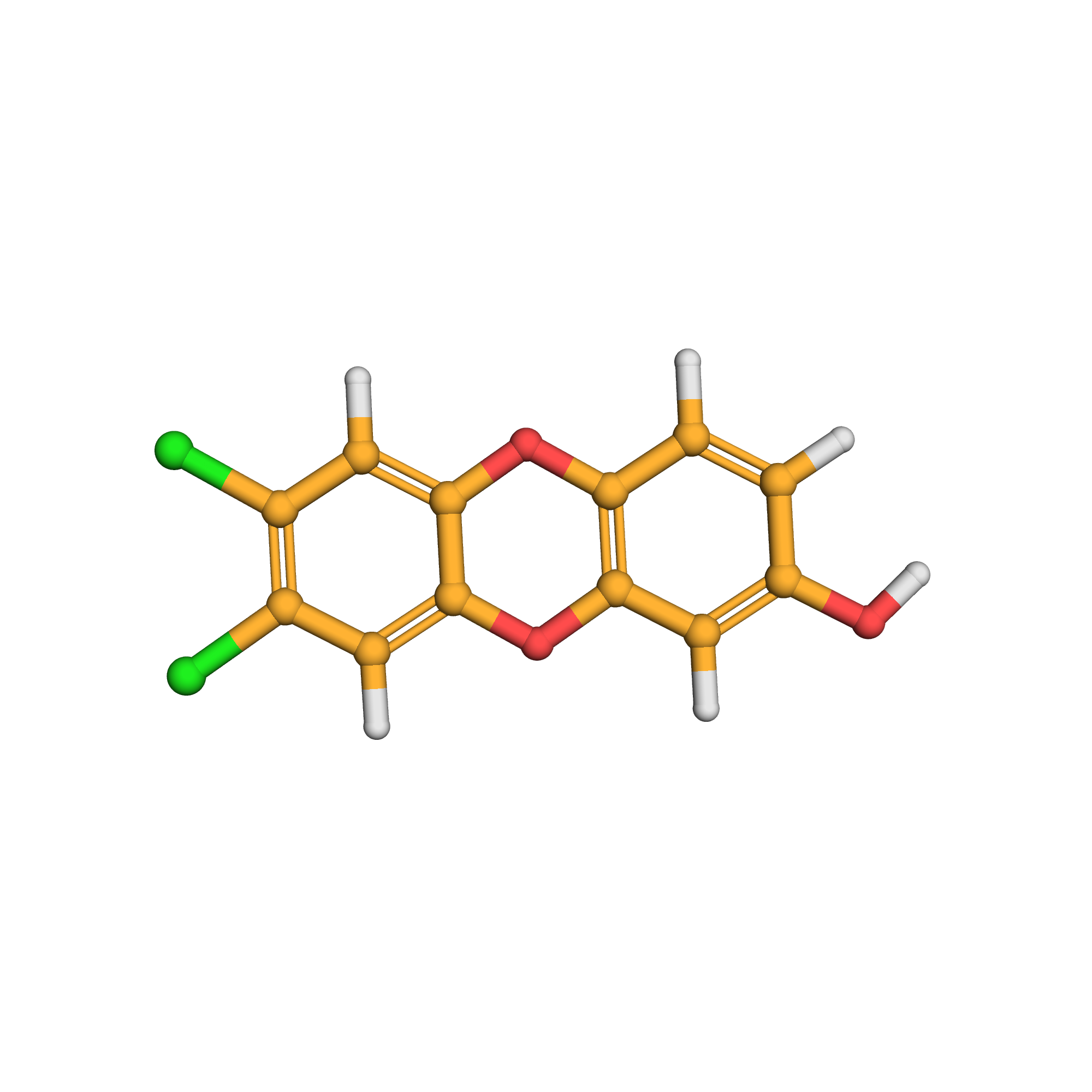
8-hydroxy-2,3-dichlorodibenzo-p-dioxin
Synonyms: "7,8-dichlorodibenzo-p-dioxin-2-ol", "2-hydroxy-7,8-dichlorodibenzo-p-dioxin", "7-hydroxy-2,3-dichlorodibenzo-p-dioxin", "2,3-dichlorodibenzo-p-dioxin-8-ol", "2-hydroxy-7,8-dichloro dibenzo-p-dioxine".
Source: 8-Hydroxy-2,3-dichlorodibenzo-p-dioxin is a metabolite of polychlorinated dibenzodioxins (PCDDs). PCDDs occur as by-products in the manufacture of some organochlorines, in the incineration of chlorine-containing substances such as PVC (polyvinyl chloride), in the chlorine bleaching of paper, and from natural sources such as volcanoes and forest fires.
Identifiers:
IUPAC Name: 7,8-dichlorodibenzo-p-dioxin-2-ol
CAS Number: 97741-80-5
PubChem ID: 176661
InChiKey: OHQHUADMUDBNSM-UHFFFAOYSA-N
Canonical SMILES: C1=CC2=C(C=C1O)OC3=CC(=C(C=C3O2)Cl)Cl
Structural Properties:
Molecular Formula: C12H6Cl2O3
Molecular Weight: 269.077
Pharmacophore Features:
Number of bond donors: 1
Number of bond acceptors: 3
Number of atoms different from hydrogen: 17
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Kester MH, Bulduk S, van Toor H, Tibboel D, Meinl W, Glatt H, Falany CN, Coughtrie MW, Schuur AG, Brouwer A, Visser TJ. 2002. Potent inhibition of estrogen sulfotransferase by hydroxylated metabolites of polyhalogenated aromatic hydrocarbons reveals alternative mechanism for estrogenic activity of endocrine disrupters. J Clin Endocrinol Metab 87(3):1142-1150. DOI: 10.1210/jcem.87.3.8311. URL: https://academic.oup.com/jcem/article/87/3/1142/2846988.
External Links





2D-structure
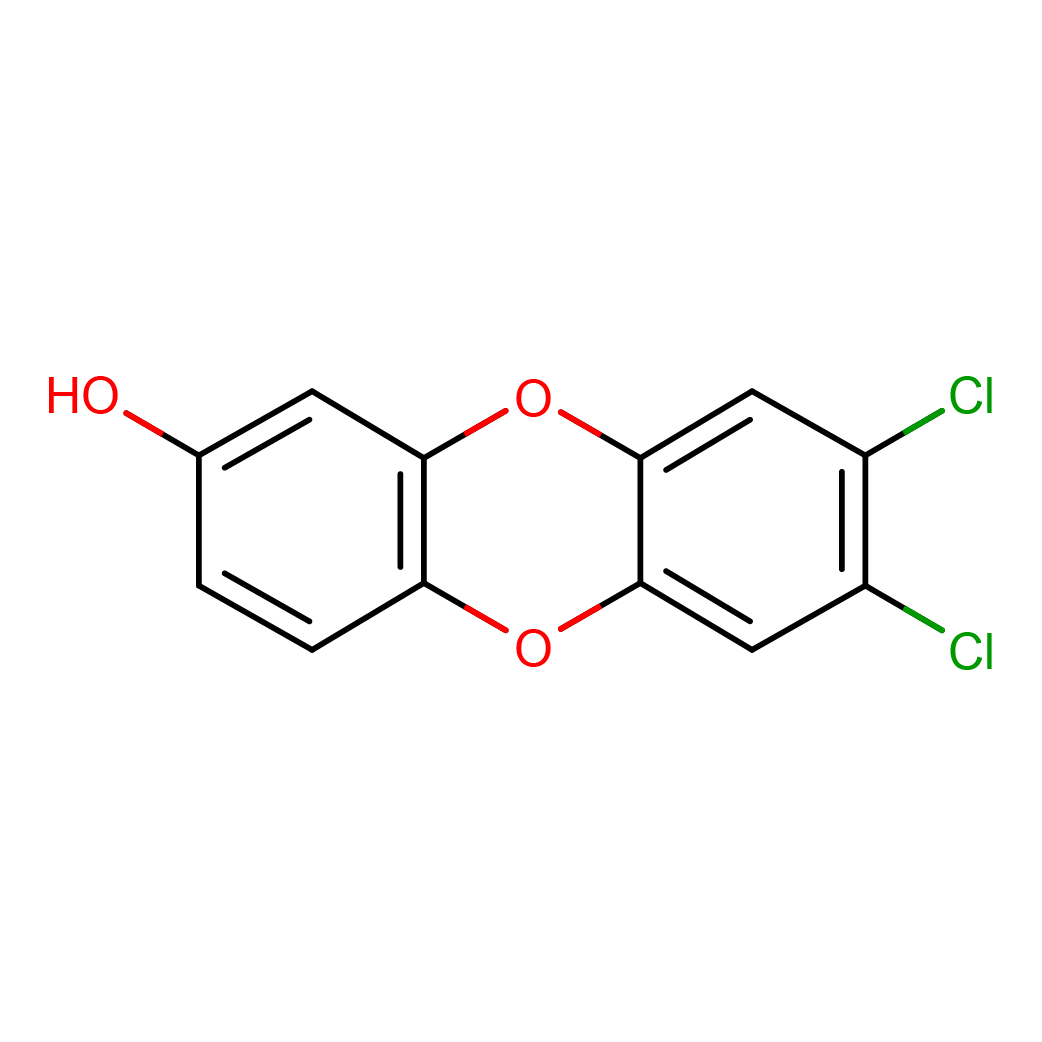
3D-structure