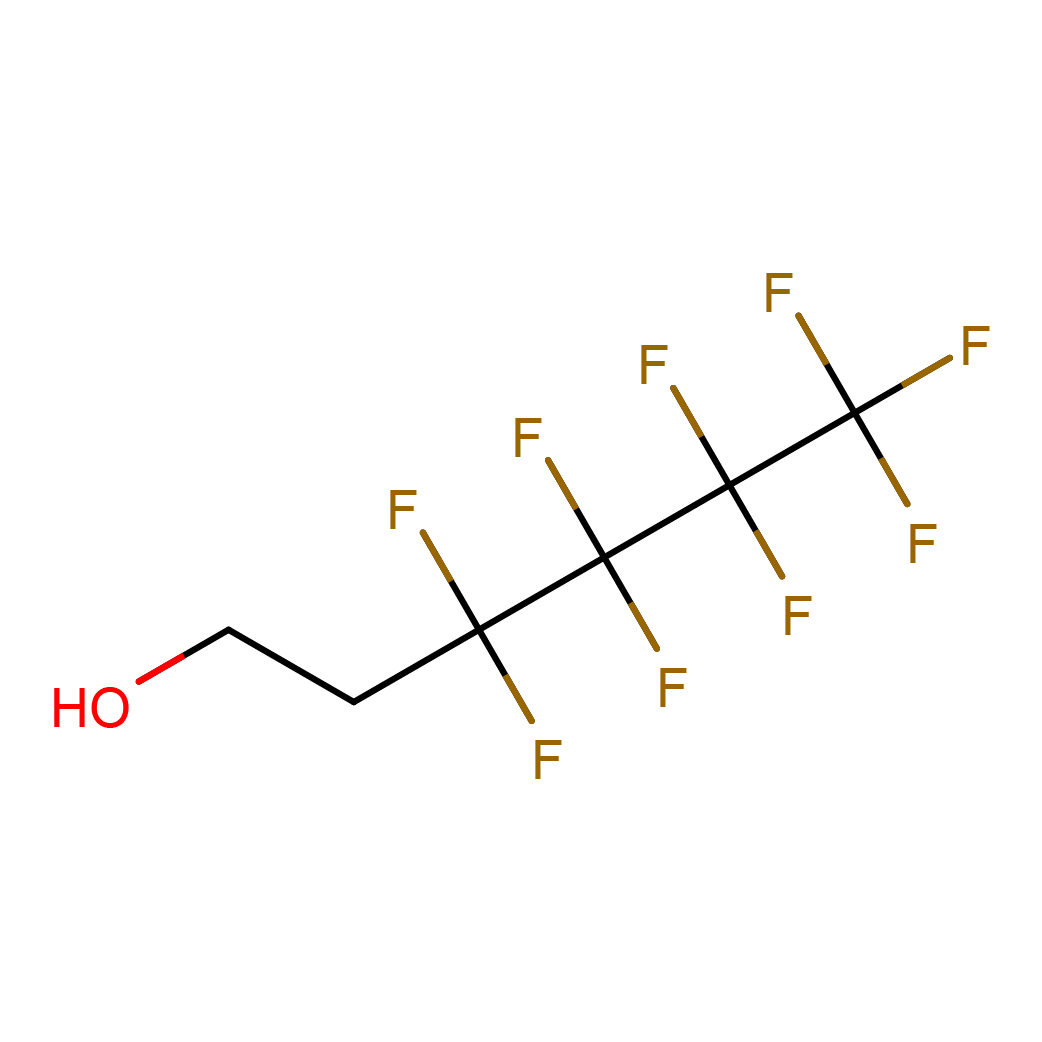
1-hexanol, 3,3,4,4,5,5,6,6,6-nonafluoro-
Synonyms: "1H,1H,2H,2H-perfluorohexan-1-ol", "2-(perfluorobutyl)ethanol", "3,3,4,4,5,5,6,6,6-nonafluorohexan-1-ol", "1H,1H,2H,2H-nonafluoro-1-hexanol", "3,3,4,4,5,5,6,6,6-nonafluorohexanol", "1,1,2,2-tetrahydroperfluoro-1-hexanol", "1H,1H,2H,2H-perfluorohexanol", "1H,1H,2H,2H-nonafluorohexan-1-ol", "2-perfluorobutylethanol".
Source: 3,3,4,4,5,5,6,6,6-nonafluorohexan-1-ol is a solvent.
Identifiers:
IUPAC Name: 3,3,4,4,5,5,6,6,6-nonafluorohexan-1-ol
CAS Number: 2043-47-2
PubChem ID: 74883
InChiKey: JCMNMOBHVPONLD-UHFFFAOYSA-N
Canonical SMILES: C(CO)C(C(C(C(F)(F)F)(F)F)(F)F)(F)F
Structural Properties:
Molecular Formula: C6H5F9O
Molecular Weight: 264.091
Pharmacophore Features:
Number of bond donors: 1
Number of bond acceptors: 10
Number of atoms different from hydrogen: 16
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Liu C, Du Y and Zhou B. 2007. Evaluation of estrogenic activities and mechanism of action of perfluorinated chemicals determined by vitellogenin induction in primary cultured tilapia hepatocytes. Aquatic toxicology 85(4):267-277. DOI: 10.1016/j.aquatox.2007.09.009. URL: https://www.ncbi.nlm.nih.gov/pubmed/17980923.
Rosenmai AK, Taxvig C, Svingen T, Trier X, Vugt?Lussenburg BMA, Pedersen M, Lesnaİ L, Jaİgou B and Vinggaard AM. 2016. Fluorinated alkyl substances and technical mixtures used in food paper?packaging exhibit endocrine?related activity in vitro. Andrology 4(4):662-672. DOI: 10.1111/andr.12190. URL: https://www.ncbi.nlm.nih.gov/pubmed/27152447.
External Links

2D-structure
3D-structure