2-mercaptobenzothiazole
Synonyms: "2-mercaptobenzothiazole", "2-benzothiazolethiol", "captax", "Benzothiazolethiol", "Benzo[d]thiazole-2-thiol", "1,3-benzothiazole-2-thiol", "benzothiazole-2-thiol", "2(3H)-benzothiazolethione", "benzo[d]thiazole-2(3H)-thione", "rotax", "accelerator M", "dermacid", "sulfadene", "thiotax", "kaptax", "mertax", "rokon", "vulkacit M", "Ekagom G", "Accel M", "2-MBT", "mebithizol", "mebetizole", "Kaptaks", "Nuodeb 84", "Soxinol M", "Vulkacit Mercapto", "Pneumax MBT", "2-Mercaptobenzthiazole", "Royal MBT", "Mercaptobenzthiazole", "Mercaptobenzothiazol", "Vulkacit Mercapto/C".
Source: 2-mercaptobenzothiazole is principally used as a reactant in the manufacture of rubber products, but is also used as a corrosion inhibitor in oils, greases and cooling fluids. It is addedto polyether polymers as a stabilizer to resist damage by air and ozone
Identifiers:
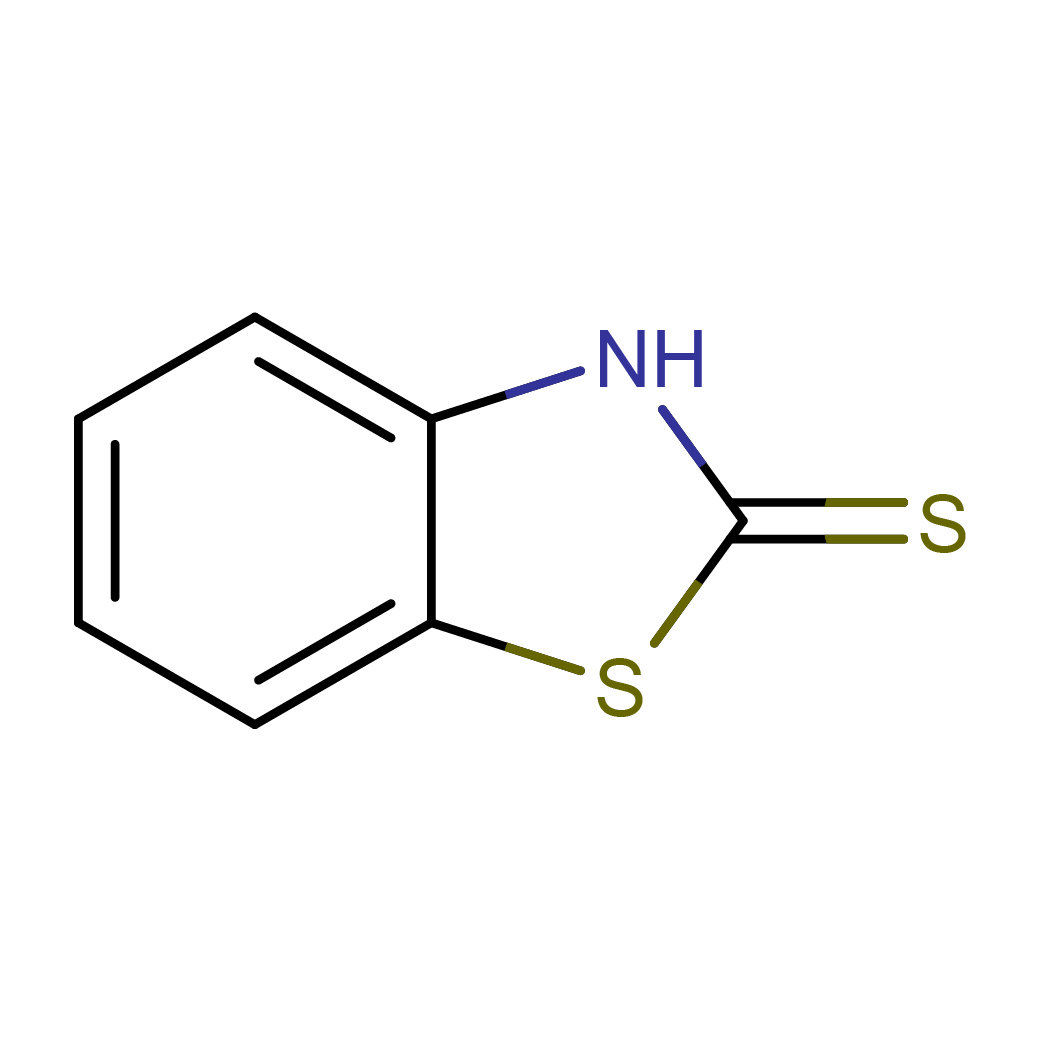
IUPAC Name: 3H-1,3-benzothiazole-2-thione
CAS Number: 149-30-4
PubChem ID: 697993
InChiKey: YXIWHUQXZSMYRE-UHFFFAOYSA-N
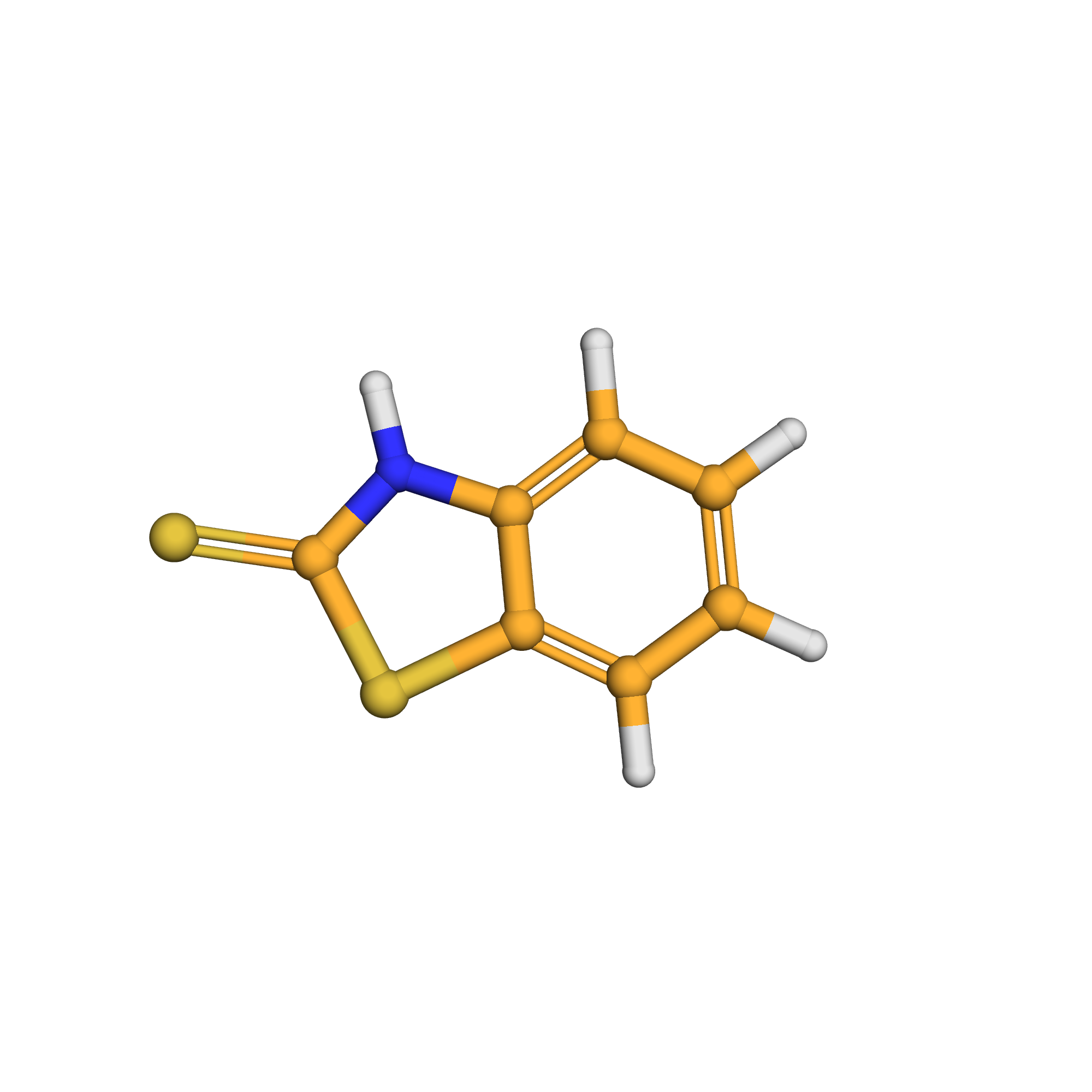
Canonical SMILES: C1=CC=C2C(=C1)NC(=S)S2
Structural Properties:
Molecular Formula: C7H5NS2
Molecular Weight: 167.244
Pharmacophore Features:
Number of bond donors: 1
Number of bond acceptors: 2
Number of atoms different from hydrogen: 10
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Hornung, M.W., Kosian, P.A., Haselman, J.T., Korte, J.J., Challis, K., Macherla, C., Nevalainen, E. and Degitz, S.J., 2015. In vitro, ex vivo, and in vivo determination of thyroid hormone modulating activity of benzothiazoles. Toxicological Sciences, 146(2), pp.254-264. DOI: https://doi.org/10.1093/toxsci/kfv090. URL: https://academic.oup.com/toxsci/article/146/2/254/1654868.
Nelson, K.R., Schroeder, A.L., Ankley, G.T., Blackwell, B.R., Blanksma, C., Degitz, S.J., Flynn, K.M., Jensen, K.M., Johnson, R.D., Kahl, M.D. and Knapen, D., 2016. Impaired anterior swim bladder inflation following exposure to the thyroid peroxidase inhibitor 2-mercaptobenzothiazole part I: Fathead minnow. Aquatic Toxicology, 173, pp.192-203. DOI: https://doi.org/10.1016/j.aquatox.2015.12.024. URL: https://www.sciencedirect.com/science/article/pii/S0166445X15301399.
Stinckens, E., Vergauwen, L., Schroeder, A.L., Maho, W., Blackwell, B.R., Witters, H., Blust, R., Ankley, G.T., Covaci, A., Villeneuve, D.L. and Knapen, D., 2016. Impaired anterior swim bladder inflation following exposure to the thyroid peroxidase inhibitor 2-mercaptobenzothiazole part II: Zebrafish. Aquatic Toxicology, 173, pp.204-217. DOI: https://doi.org/10.1016/j.aquatox.2015.12.023. URL: https://www.sciencedirect.com/science/article/pii/S0166445X15301387.
Tietge, J.E., Degitz, S.J., Haselman, J.T., Butterworth, B.C., Korte, J.J., Kosian, P.A., Lindberg-Livingston, A.J., Burgess, E.M., Blackshear, P.E. and Hornung, M.W., 2013. Inhibition of the thyroid hormone pathway in Xenopus laevis by 2-mercaptobenzothiazole. Aquatic toxicology, 126, pp.128-136. DOI: https://doi.org/10.1016/j.aquatox.2012.10.013. URL: https://www.sciencedirect.com/science/article/pii/S0166445X12002949.
External Links


2D-structure
3D-structure