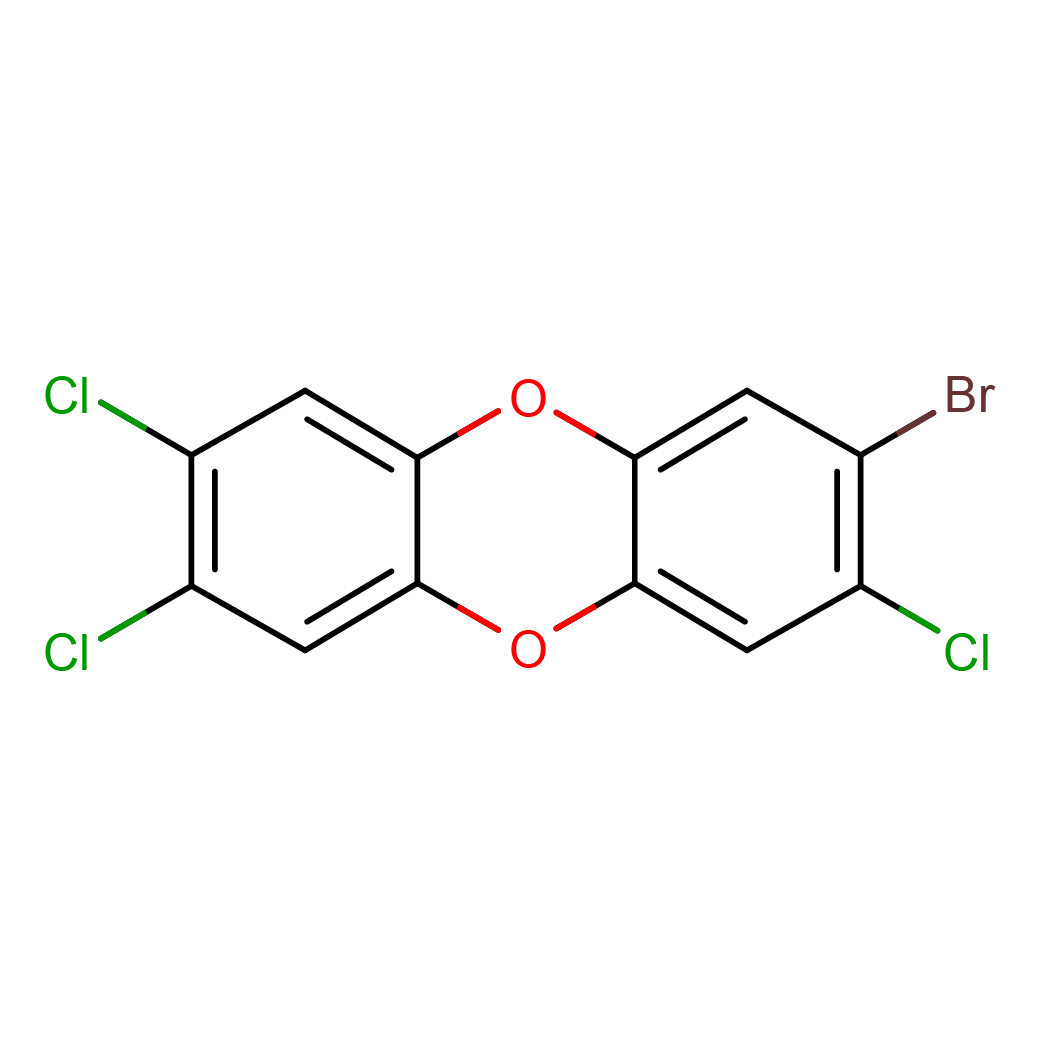
2-bromo-3,7,8-trichlorodibenzo-p-dioxin
Synonyms: "2-bromo,3,7,8-trichloro-dibenzo-dioxin", "2-bromo-3,7,8-trichlorodibenzo(b,e)(1,4)dioxin", "2-bromo-3,7,8-trichlorodibenzo[b,e][1,4]dioxin".
Source: 2-bromo-3,7,8-trichlorodibenzo-p-dioxin is a mixed bromo/chloro congener of dibenzodioxins, which is produced during the incineration of these chemicals.
Identifiers:
IUPAC Name: 2-bromo-3,7,8-trichlorodibenzo-p-dioxin
CAS Number: 109333-33-7
PubChem ID: 114896
InChiKey: MEDUBLZGNFZMKG-UHFFFAOYSA-N
Canonical SMILES: C1=C2C(=CC(=C1Cl)Cl)OC3=CC(=C(C=C3O2)Cl)Br
Structural Properties:
Molecular Formula: C12H4BrCl3O2
Molecular Weight: 366.416
Pharmacophore Features:
Number of bond donors: 0
Number of bond acceptors: 2
Number of atoms different from hydrogen: 18
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Hornung MW, Zabel EW, Peterson RE. 1996. Toxic equivalency factors of polybrominated dibenzo-p-dioxin, dibenzofuran, biphenyl, and polyhalogenated diphenyl ether congeners based on rainbow trout early life stage mortality. Toxicol Appl Pharmacol 140(2):227-234. DOI: 10.1006/taap.1996.0217. URL: https://www.sciencedirect.com/science/article/pii/S0041008X96902173.
Mason G, Zacharewski T, Denomme MA, Safe L, Safe S. 1987. Polybrominated dibenzo-p-dioxins and related compounds: Quantitative in vivo and in vitro structure-activity relationships. Toxicology 44(3):245-255. DOI: 10.1016/0300-483X(87)90027-8. URL: https://www.sciencedirect.com/science/article/pii/0300483X87900278.
Schulz-Schalge T, Koch E, Schwind KH, Hutzinger O, Neubert D. 1991. Inductive potency of TCDD, TBDD and three 2,3,7,8-mixed-halogenated dioxins in liver microsomes of male rats. Enzyme kinetic considerations. Chemosphere 23(11-12):1925-1931. DOI: 10.1016/0045-6535(91)90040-K. URL: https://www.sciencedirect.com/science/article/pii/004565359190040K.
External Links

2D-structure
3D-structure