2,4,6-tri-tert-butylphenol
Synonyms: "2,4,6-tri-tert-butylphenol", "Voidox", "Alkofen B", "2,4,6-Tris(tert-butyl)phenol", "2,4,6-Tri-t-butylphenol", "2,4,6-tritert-butylphenol", "2,4,6-tris(1,1-dimethylethyl)phenol", "P 23", "tris(1,1-dimethylethyl)phenol", "2,4,6-tri-tert-butyl phenol", "2,4,6-tri-tert-butyl-1-hydroxybenzene", "2,4,6-tri(tert-butyl)phenol".
Source: 2,4,6-Tri-tert-butylphenol is the starting material for the synthesis of 2,6-di-tert-butyl-4-methoxyphenol which is a powerful antioxidant.
Identifiers:
IUPAC Name: 2,4,6-tritert-butylphenol
CAS Number: 732-26-3
PubChem ID: 12902
InChiKey: PFEFOYRSMXVNEL-UHFFFAOYSA-N
Canonical SMILES: CC(C)(C)C1=CC(=C(C(=C1)C(C)(C)C)O)C(C)(C)C
Structural Properties:
Molecular Formula: C18H30O
Molecular Weight: 262.437
Pharmacophore Features:
Number of bond donors: 1
Number of bond acceptors: 1
Number of atoms different from hydrogen: 19
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Tollefsen KE, Eikvar S, Finne EF, Fogelberg O, Gregersen IK. 2008. Estrogenicity of alkylphenols and alkylated non-phenolics in a rainbow trout (Oncorhynchus mykiss) primary hepatocyte culture. Ecotoxicol Environ Saf 71(2):370-383. DOI: 10.1016/j.ecoenv.2007.10.006. URL: https://www.sciencedirect.com/science/article/pii/S0147651307002400?via%3Dihub.
External Links

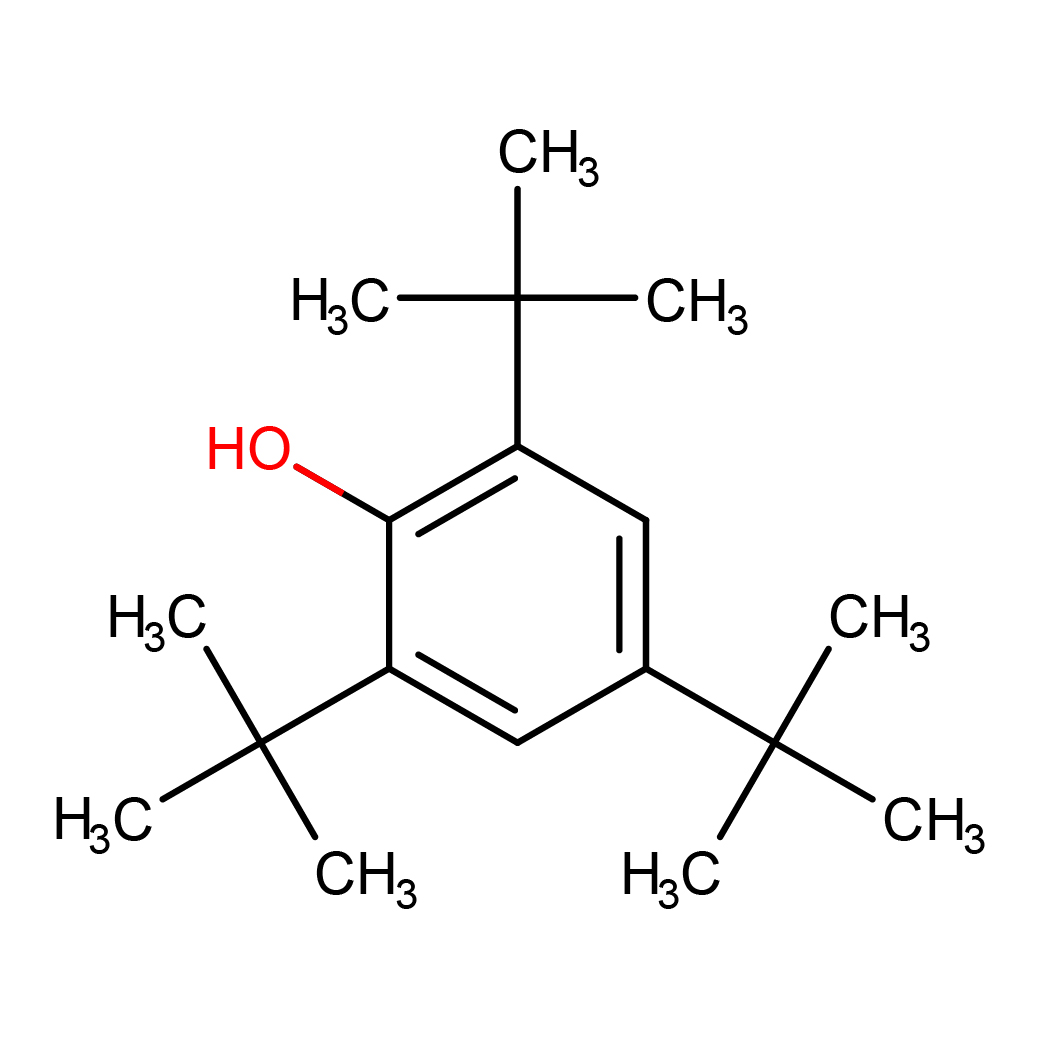
2D-structure
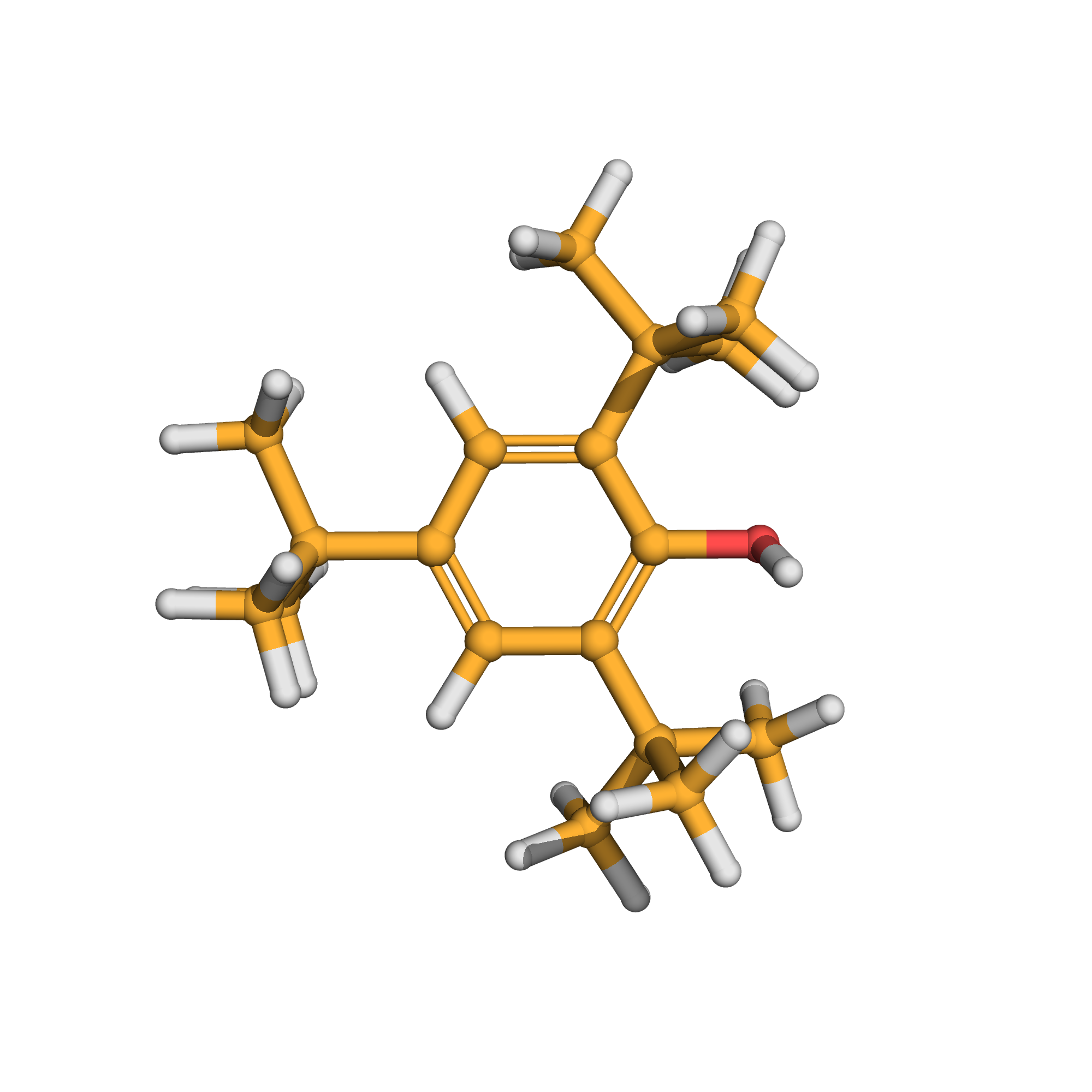
3D-structure