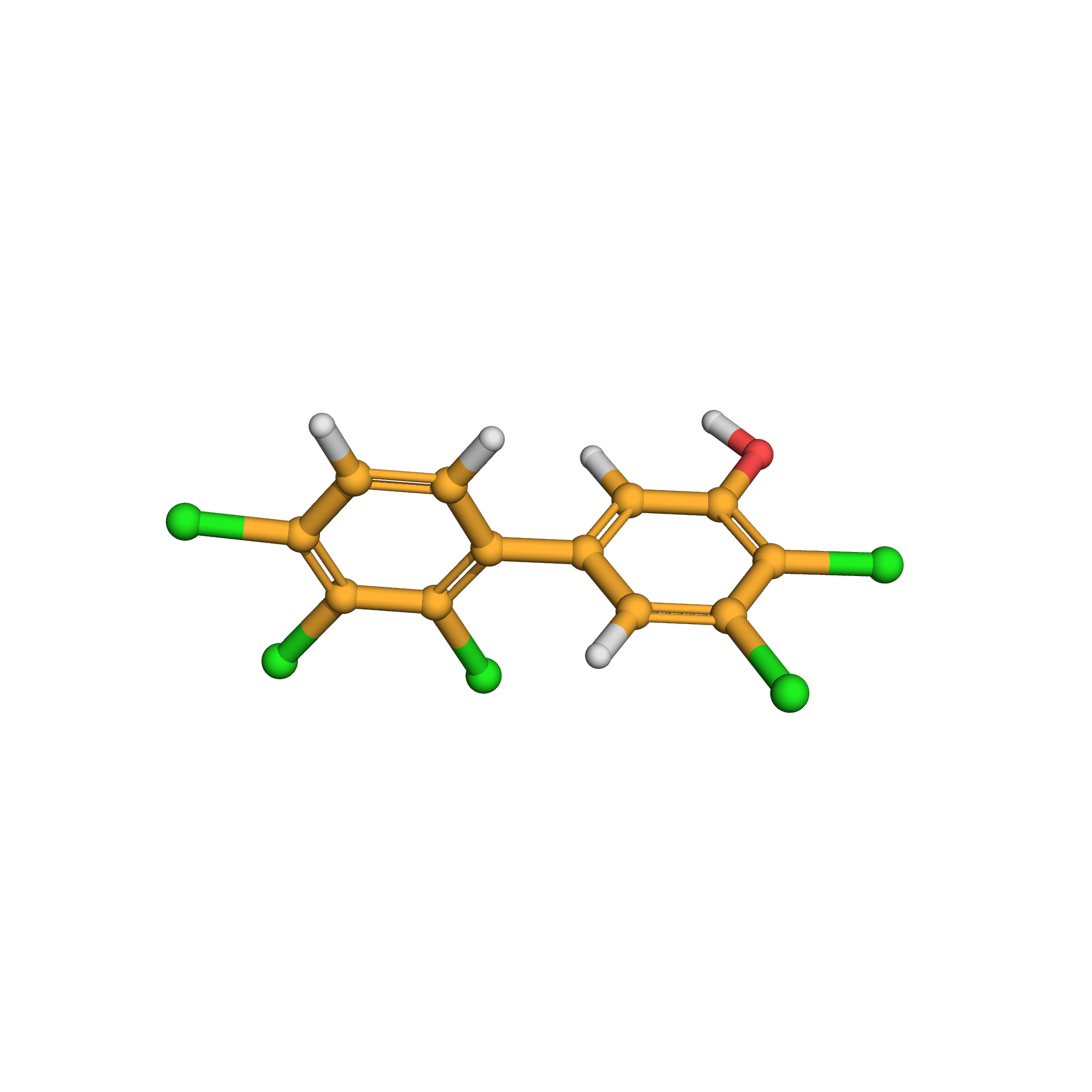
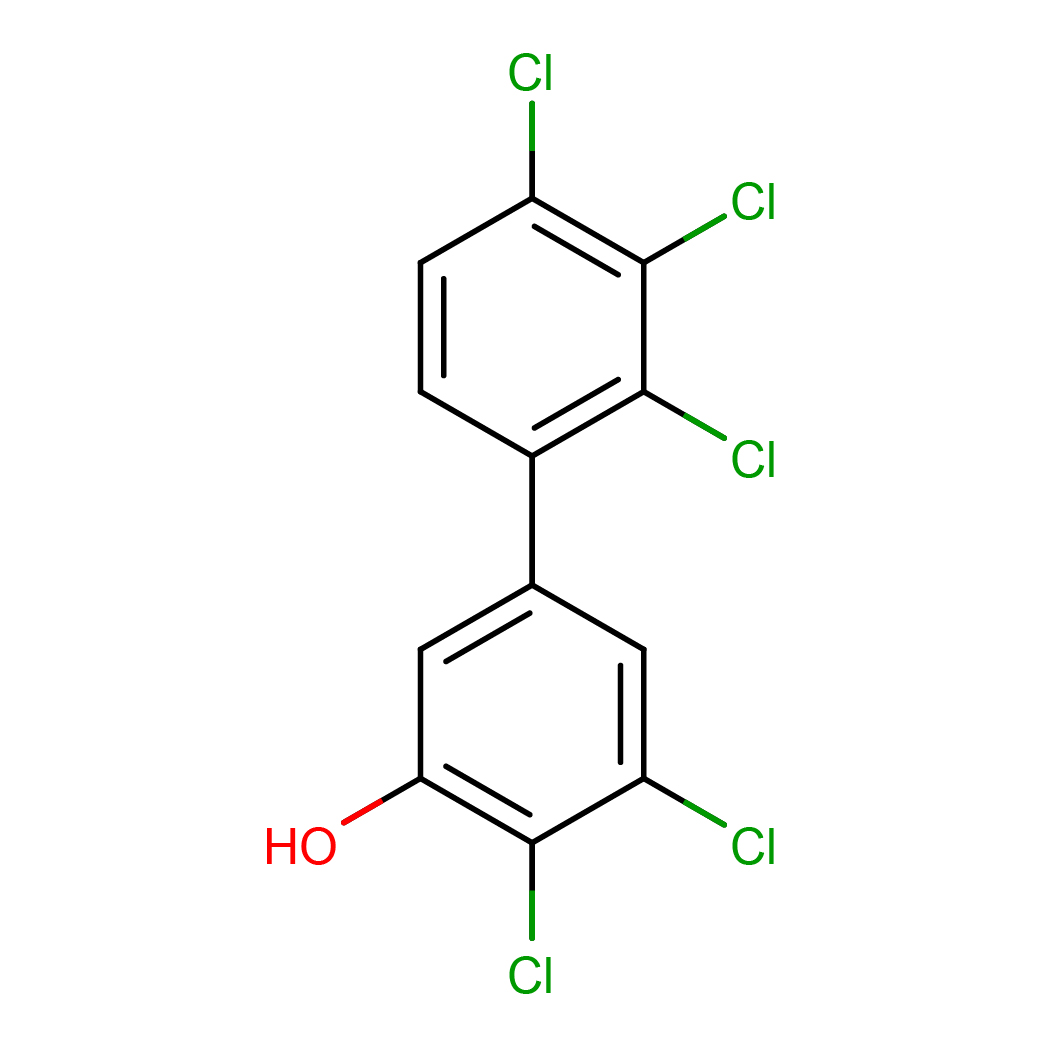
2,3-dichloro-5-(2,3,4-trichlorophenyl)phenol
Synonyms: "2,3-dichloro-5-(2,3,4-trichlorophenyl)phenol","2',3',4,4',5-pentachloro-(1,1'-biphenyl)-3-ol", "2',3,3',4,4'-pentachloro-1,1'-biphenyl-5-ol","5'-hydroxy-2,3,3',4,4'-pentachlorobiphenyl".
Source: 2,3-dichloro-5-(2,3,4-trichlorophenyl)phenol is a metabolite of polybrominated diphenyl ethers, which are widely used as flame retardants.
Identifiers:
IUPAC Name: 2,3-dichloro-5-(2,3,4-trichlorophenyl)phenol
CAS Number: 150975-81-8
PubChem ID: 177911
InChiKey: PBGSKHGHSJAIAU-UHFFFAOYSA-N
Canonical SMILES: C1=CC(=C(C(=C1C2=CC(=C(C(=C2)Cl)Cl)O)Cl)Cl)Cl
Structural Properties:
Molecular Formula: C12H5Cl5O
Molecular Weight: 342.421
Pharmacophore Features:
Number of bond donors: 1
Number of bond acceptors: 1
Number of atoms different from hydrogen: 18
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Kester MH, Bulduk S, Tibboel D, Meinl W, Glatt H, Falany CN, Coughtrie MW, Bergman A, Safe SH, Kuiper GG, Schuur AG, Brouwer A, Visser TJ. 2000 . Potent inhibition of estrogen sulfotransferase by hydroxylated PCB metabolites: a novel pathway explaining the estrogenic activity of PCBs. Endocrinology 141(5):1897?1900. DOI: 10.1210/endo.141.5.7530. URL: https://academic.oup.com/endo/article/141/5/1897/2988537Kramer VJ, Giesy JP. 1999. Specific binding of hydroxylated polychlorinated biphenyl metabolites and other substances to bovine calf uterine estrogen receptor: structure-binding relationships. Sci Total Environ 233(1-3):141-161. DOI: 10.1016/S0048-9697(99)00221-1. URL: https://www.sciencedirect.com/science/article/pii/S0048969799002211.
External Links




2D-structure
3D-structure