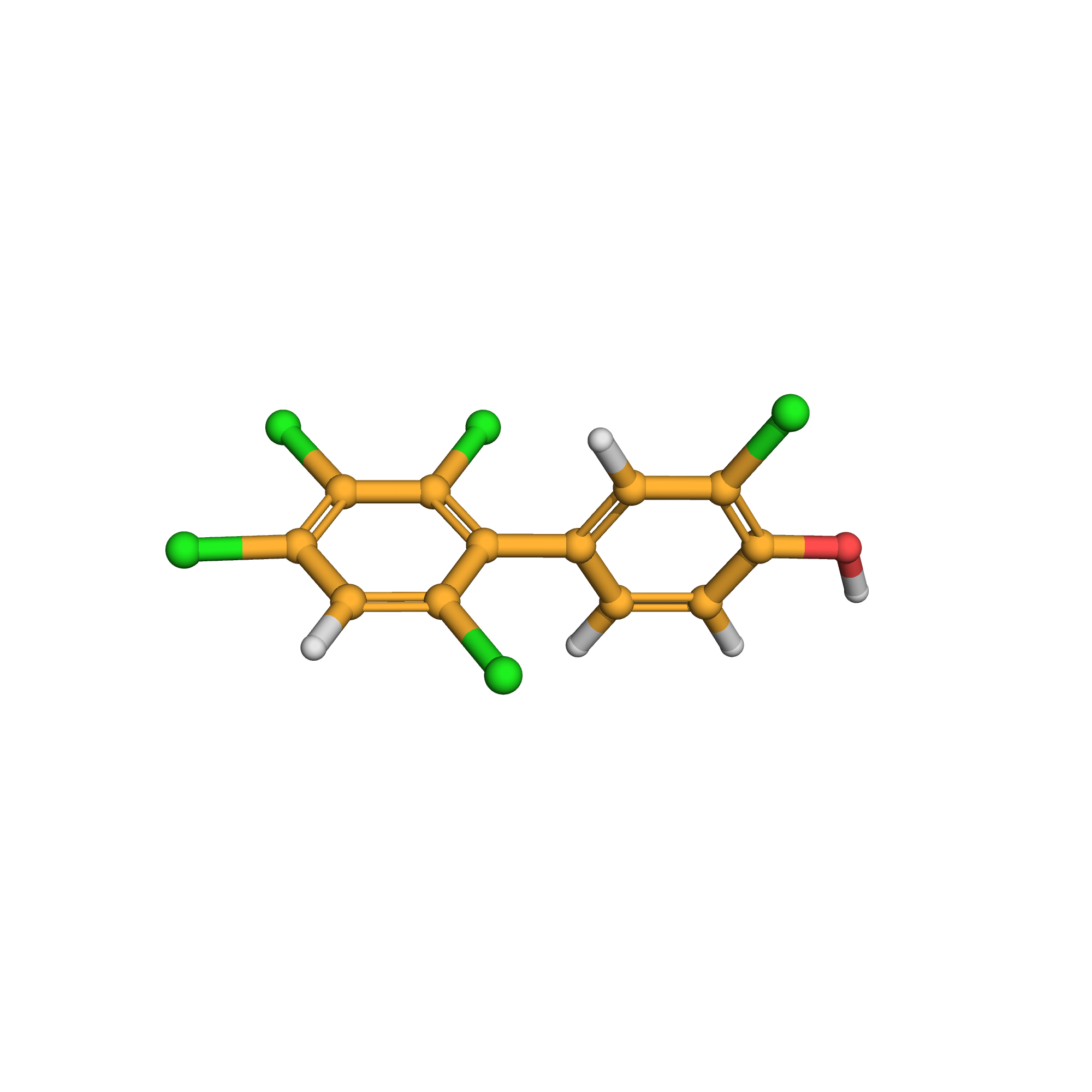
2',3,3',4',6'-pentachloro-4-biphenylol
Synonyms: "4-hydroxy-2',3,3',4',6'-pentachlorobiphenyl", "2-chloro-4-(2,3,4,6-tetrachlorophenyl)phenol".
Source: 2',3,3',4',6'-pentachloro-4-biphenylol belongs to the hydroxylated metabolites of polychlorinated biphenyls (OH-PCBs).
Identifiers:
IUPAC Name: 2-chloro-4-(2,3,4,6-tetrachlorophenyl)phenol
CAS Number: 192190-10-6
PubChem ID: 644181
InChiKey: QLPPOBFMEBKBOZ-UHFFFAOYSA-N
Canonical SMILES: C1=CC(=C(C=C1C2=C(C(=C(C=C2Cl)Cl)Cl)Cl)Cl)O
Structural Properties:
Molecular Formula: C12H5Cl5O
Molecular Weight: 342.421
Pharmacophore Features:
Number of bond donors: 1
Number of bond acceptors: 1
Number of atoms different from hydrogen: 18
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Connor K, Ramamoorthy K, Moore M, Mustain M, Chen I, Safe S, Zacharewski T, Gillesby B, Joyeux A, Balaguer P. 1997. Hydroxylated polychlorinated biphenyls (PCBs) as estrogens and antiestrogens: Structure-activity relationships. Toxicol Appl Pharmacol 145(1):111-123. DOI: 10.1006/taap.1997.8169. URL: http://www.sciencedirect.com/science/article/pii/S0041008X97981692?via%3DihubKester MH, Bulduk S, Tibboel D, Meinl W, Glatt H, Falany CN, Coughtrie MW, Bergman A, Safe SH, Kuiper GG, Schuur AG, Brouwer A, Visser TJ. 2000 . Potent inhibition of estrogen sulfotransferase by hydroxylated PCB metabolites: a novel pathway explaining the estrogenic activity of PCBs. Endocrinology 141(5):1897?1900. DOI: 10.1210/endo.141.5.7530. URL: https://academic.oup.com/endo/article/141/5/1897/2988537.
External Links


2D-structure
3D-structure