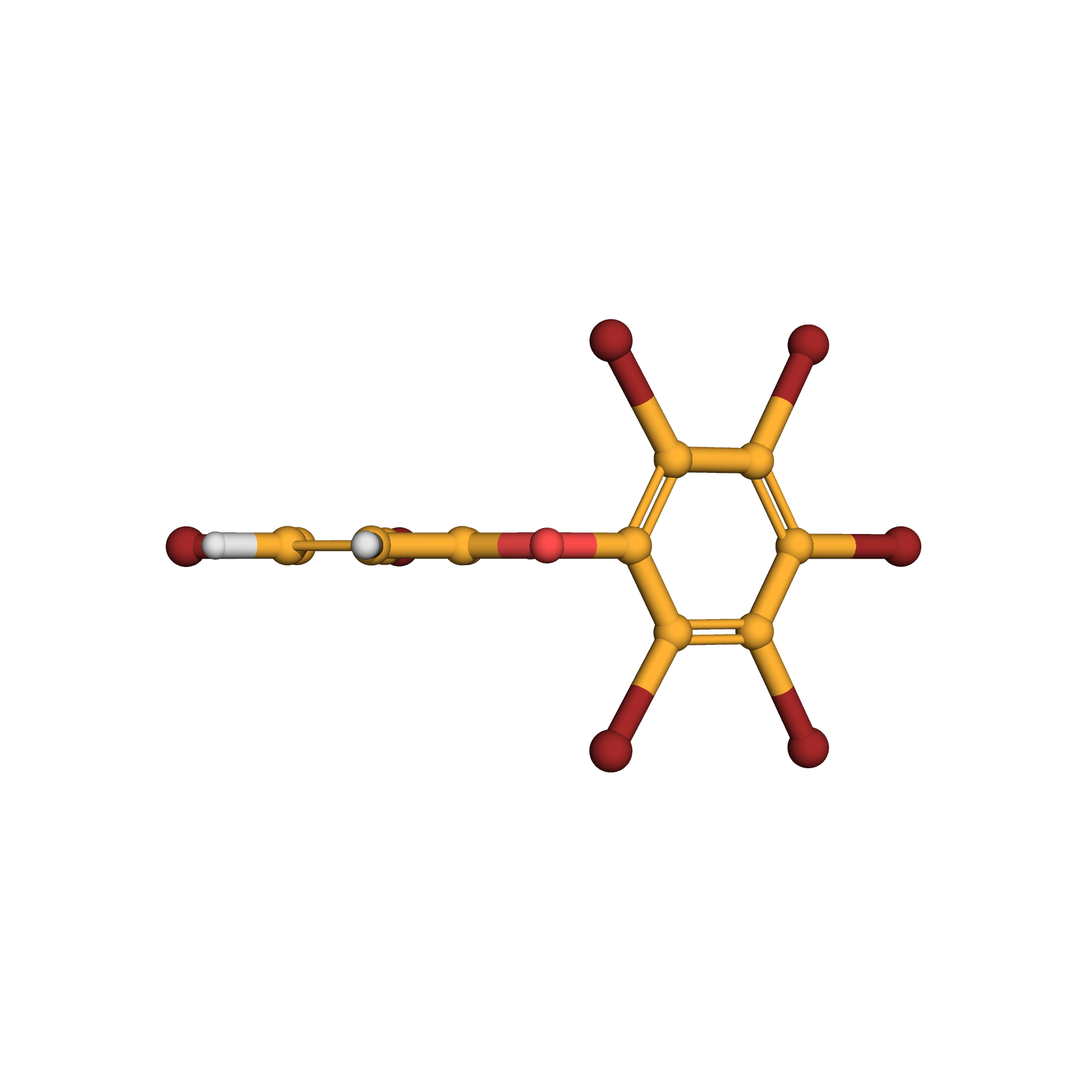
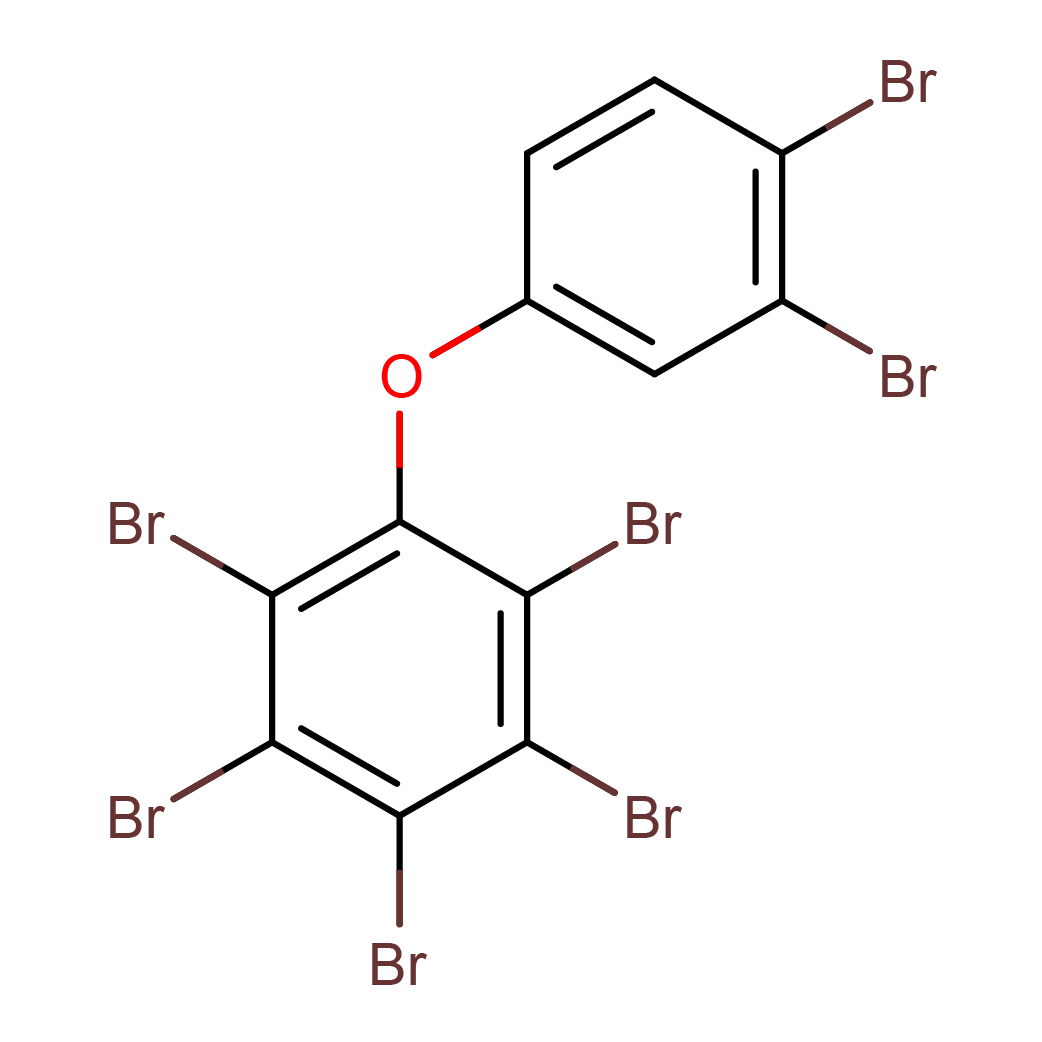
2,3,3',4,4',5,6-heptabromodiphenyl ether
Synonyms: "PBDE No. 190", "2,3,3',4,4,',5,6-heptabromodiphenyl ether", "BDE-190", "2,3,3,4,4,5,6-heptabromodiphenyl ether".
Source: 2,3,3',4,4',5,6-heptabromodiphenyl ether is a PBDE (Polybrominated diphenyl ethers) congener. PBDEs are flame-retardant man-made chemicals found in plastics used in a variety of consumer products to make them difficult to burn.
Identifiers:
IUPAC Name: 1,2,3,4,5-pentabromo-6-(3,4-dibromophenoxy)benzene
CAS Number: 189084-68-2
PubChem ID: 12110099
InChiKey: OUEYHQIMJGHOQN-UHFFFAOYSA-N
Canonical SMILES: C1=CC(=C(C=C1OC2=C(C(=C(C(=C2Br)Br)Br)Br)Br)Br)Br
Structural Properties:
Molecular Formula: C12H3Br7O
Molecular Weight: 722.483
Pharmacophore Features:
Number of bond donors: 0
Number of bond acceptors: 1
Number of atoms different from hydrogen: 20
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Hamers T, Kamstra JH, Sonneveld E, Murk AJ, Kester MH, Andersson PL, Legler J, Brouwer A. 2006. In vitro profiling of the endocrine-disrupting potency of brominated flame retardants. Toxicol Sci 92(1):157-173. DOI: 10.1093/toxsci/kfj187. URL: https://academic.oup.com/toxsci/article/92/1/157/1642946Meerts IATM, Letcher RJ, Hoving S, Marsh G, Bergman a?, Lemmen JG, van der Burg B, Brouwer A. 2001. In vitro estrogenicity of polybrominated diphenyl ethers, hydroxylated PBDEs, and polybrominated bisphenol A compounds. Environ Health Perspect 109(4):399-407. DOI: 10.2307/3454900 . URL: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1240281/.
External Links

2D-structure
3D-structure