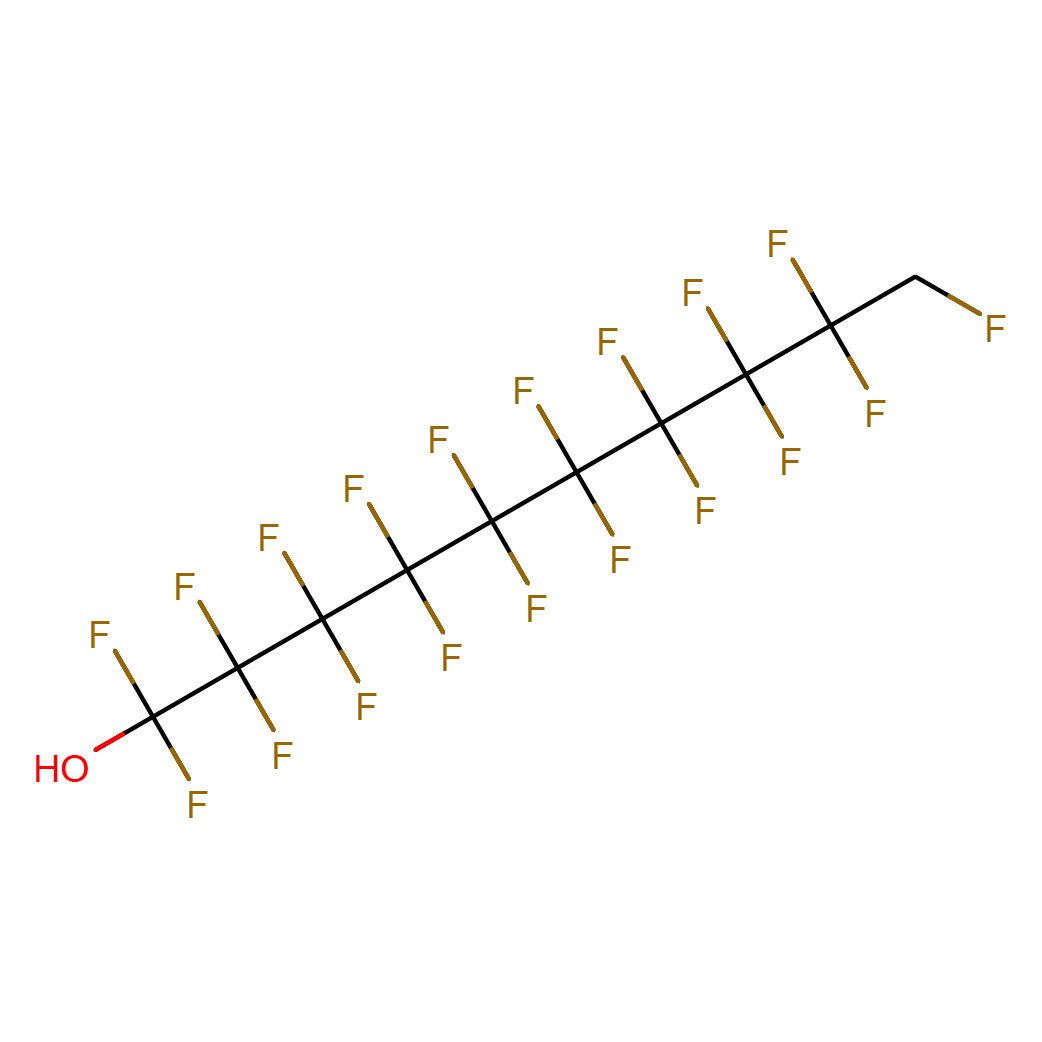
1,1,2,2,3,3,4,4,5,5,6,6,7,7,8,8,9,9,10-nonadecafluorodecan-1-ol
Synonyms: "nonadecafluoro-1-decanol", "nonadecafluoro-1-decanol".
Source: 1,1,2,2,3,3,4,4,5,5,6,6,7,7,8,8,9,9,10-nonadecafluorodecan-1-ol is a fluorotelomer alcohol (FTOHs). FTOHs are typically used as precursor compounds in the manufacture of fluorinated polymers used in paper and carpet treatments and are considered potential sources of perfluoroalkylcarboxylate (PFCA) contaminants in the environment.
Identifiers:
IUPAC Name: 1,1,2,2,3,3,4,4,5,5,6,6,7,7,8,8,9,9,10-nonadecafluorodecan-1-ol
CAS Number: N/A
PubChem ID: 53879331
InChiKey: HIRBIVIDAZKABO-UHFFFAOYSA-N
Canonical SMILES: C(C(C(C(C(C(C(C(C(C(O)(F)F)(F)F)(F)F)(F)F)(F)F)(F)F)(F)F)(F)F)(F)F)F
Structural Properties:
Molecular Formula: C10H3F19O
Molecular Weight: 500.103
Pharmacophore Features:
Number of bond donors: 1
Number of bond acceptors: 20
Number of atoms different from hydrogen: 30
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Ishibashi H, Ishida H, Matsuoka M, Tominaga N and Arizono K. 2007. Estrogenic effects of fluorotelomer alcohols for human estrogen receptor isoforms ? and ? in vitro. Biological and Pharmaceutical Bulletin 30(7):1358-1359. DOI: 10.1248/bpb.30.1358. URL: https://www.ncbi.nlm.nih.gov/pubmed/17603182.
Ishibashi H, Yamauchi R, Matsuoka M, Kim JW, Hirano M, Yamaguchi A, Tominaga N and Arizono K. 2008. Fluorotelomer alcohols induce hepatic vitellogenin through activation of the estrogen receptor in male medaka (Oryzias latipes). Chemosphere 71(10):1853-1859. DOI: 10.1016/j.chemosphere.2008.01.065. URL: https://www.ncbi.nlm.nih.gov/pubmed/18334264.
External Links



2D-structure
3D-structure