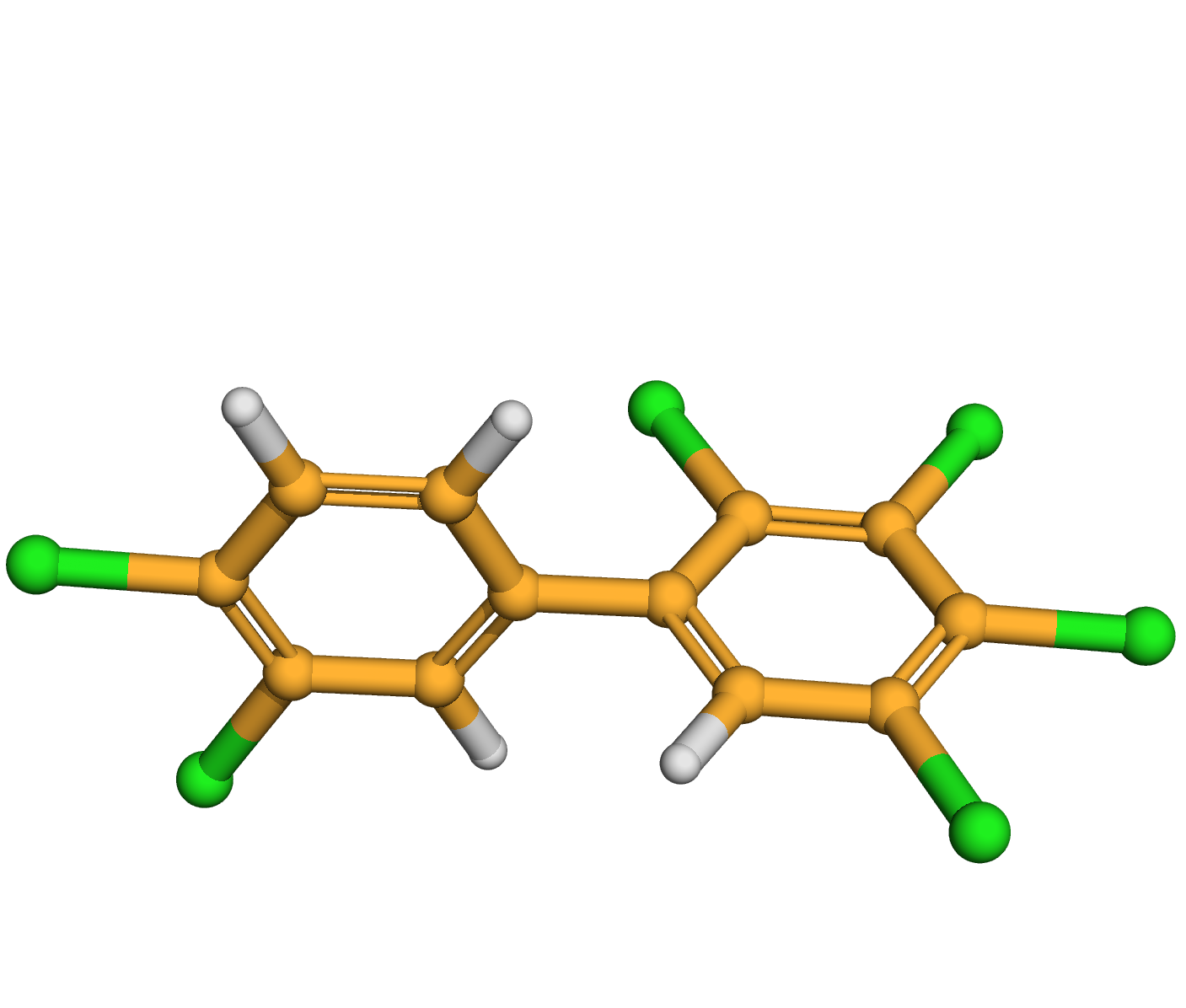
2,3,3',4,4',5-hexachlorobiphenyl
Synonyms: "2,3,4,5,3',4'-Hexachlorobiphenyl", "PCB-156", "PCB 156"
Source: 2,3,3',4,4',5-hexachlorobiphenyl is a member of the polychlorinated biphenyls (PCBs), a group of industrial chemicals used as dielectrics, coolants and lubricants in electrical equipment. PCBs contaminate water by leaching from landfills or other waste deposits.
Identifiers:
IUPAC Name: 1,2,3,4-tetrachloro-5-(3,4-dichlorophenyl)benzene
CAS Number: 38380-08-4
PubChem ID: 38019
InChiKey: LCXMEXLGMKFLQO-UHFFFAOYSA-N
Canonical SMILES: C1=CC(=C(C=C1C2=CC(=C(C(=C2Cl)Cl)Cl)Cl)Cl)Cl
Structural Properties:
Molecular Formula: C12<H4Cl6
Molecular Weight: 360.878
Pharmacophore Features:
Number of bond donors: 0
Number of bond acceptors: 0
Number of atoms different from hydrogen: 18
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Krishnan V, Safe S. 1993. Polychlorinated biphenyls (PCBs), dibenzo-p-dioxins (PCDDs), and dibenzofurans (PCDFs) as antiestrogens in MCF-7 human breast cancer cells: quantitative structure-activity relationships. Toxicol Appl Pharmacol 120(1):55-61.
Van Birgelen APJM, Smit EA, Kampen IM, Groeneveld CN, Fase KM, Van der Kolk J, Poiger H, Van den Berg M, Koeman JH, Brouwer A. 1995. Subchronic effects of 2,3,7,8-TCDD or PCBs on thyroid hormone metabolism - Use in risk assessment. European Journal of Pharmacology - Environmental Toxicology & Pharmacology Section 293(1):77-85.
External Links



2D-structure
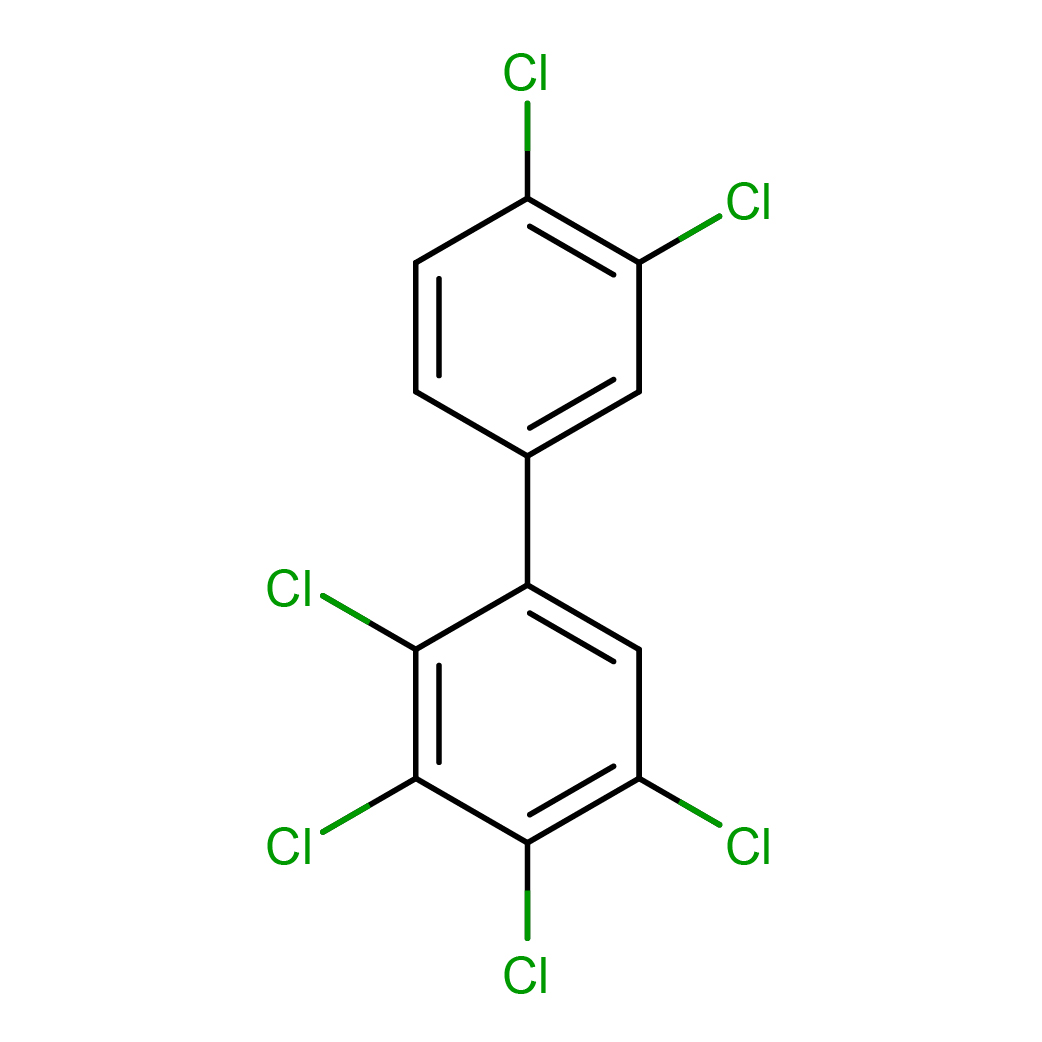
3D-structure