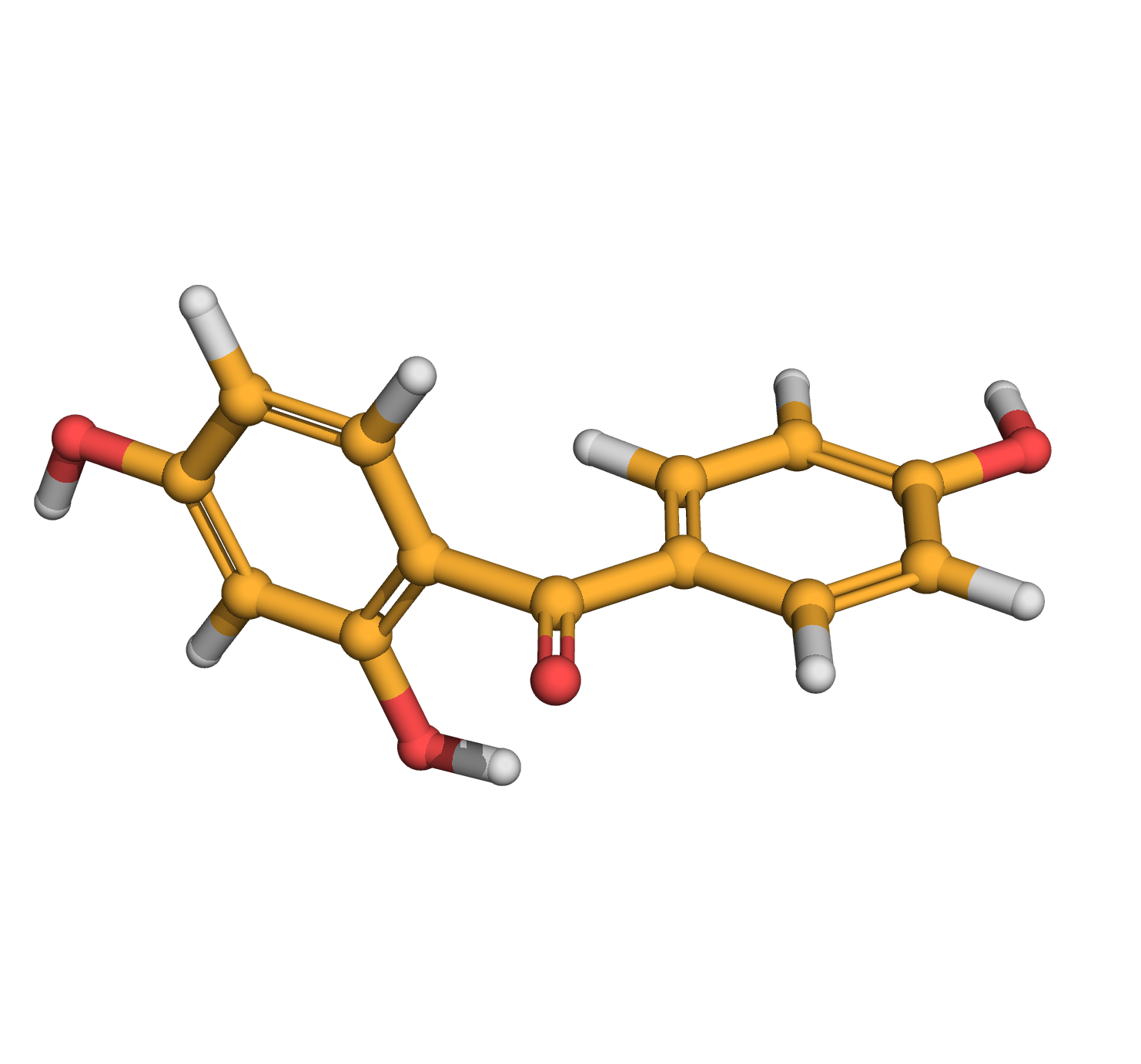
2,4,4'-trihydroxybenzophenone
Synonyms: "(2,4-dihydroxyphenyl)(4-hydroxyphenyl)methanone"
Source: 2,4,4'-trihydroxybenzophenone belong to hydroxybenzophenones, which are used as UV stabilizers in plastic surface coatings on food packaging to prevent polymer degradation and loss of quality of the packed food owing to UV light irradiation. These compounds may be transferred from the packaging to the contents, and subsequently ingested by humans. However, they are natural components of plants such as mango and muscat grape, and are also used as flavorings.
Identifiers:
IUPAC Name: (2,4-dihydroxyphenyl)-(4-hydroxyphenyl)methanone
CAS Number: 1470-79-7
PubChem ID: 73852
InChiKey: OKJFKPFBSPZTAH-UHFFFAOYSA-N
Canonical SMILES: C1=CC(=CC=C1C(=O)C2=C(C=C(C=C2)O)O)O
Structural Properties:
Molecular Formula: C13H10O4
Molecular Weight: 230.216
Pharmacophore Features:
Number of bond donors: 3
Number of bond acceptors: 4
Number of atoms different from hydrogen: 17
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Akahori Y, Nakai M, Yamasaki K, Takatsuki M, Shimohigashi Y, Ohtaki M. 2008. Relationship between the results of in vitro receptor binding assay to human estrogen receptor alpha and in vivo uterotrophic assay: Comparative study with 65 selected chemicals. Toxicol in Vitro 22(1):225-231.
Schultz TW, Seward JR, Sinks GD. 2000. Estrogenicity of benzophenones evaluated with a recombinant yeast assay: comparison of experimental and rules-based predicted activity. Environ Toxicol Chem 19(2):301-304.
External Links

2D-structure
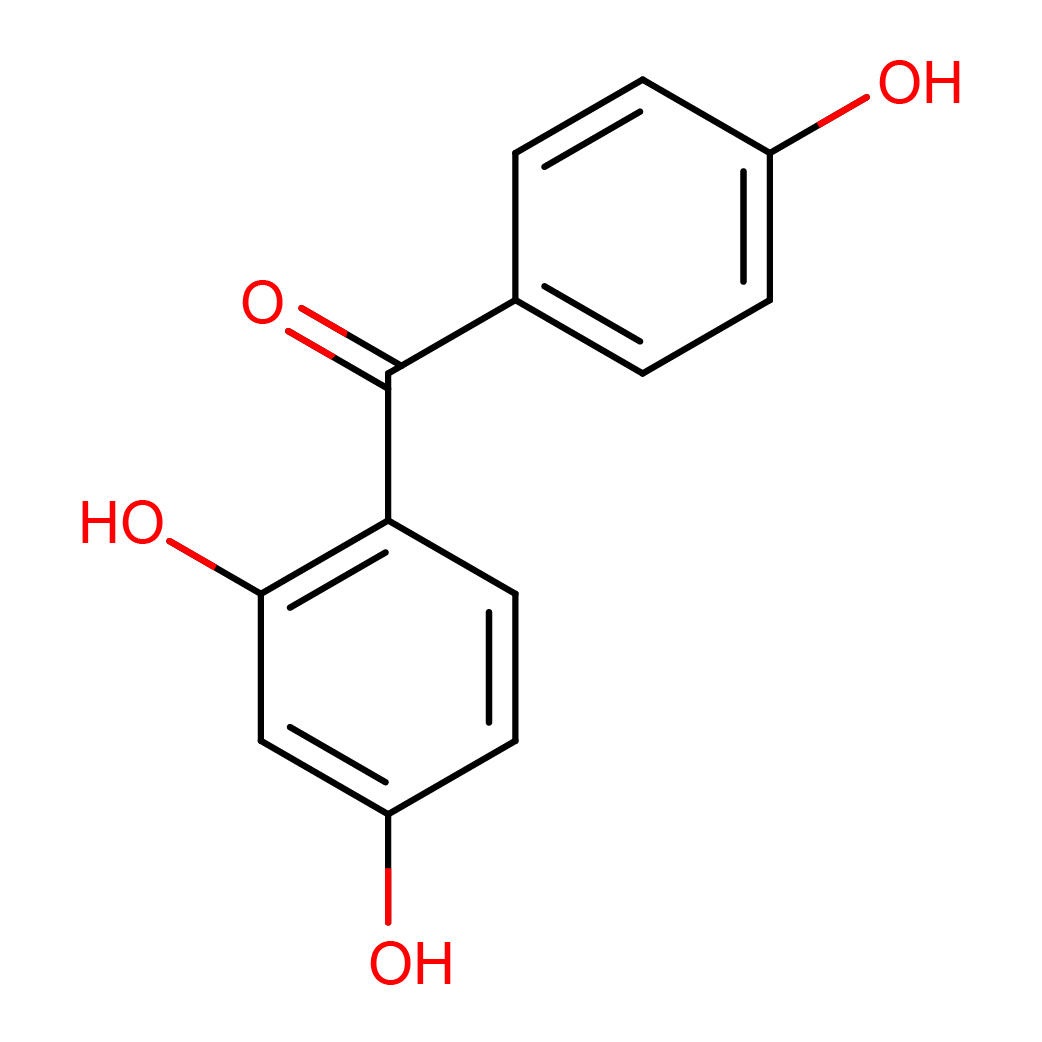
3D-structure