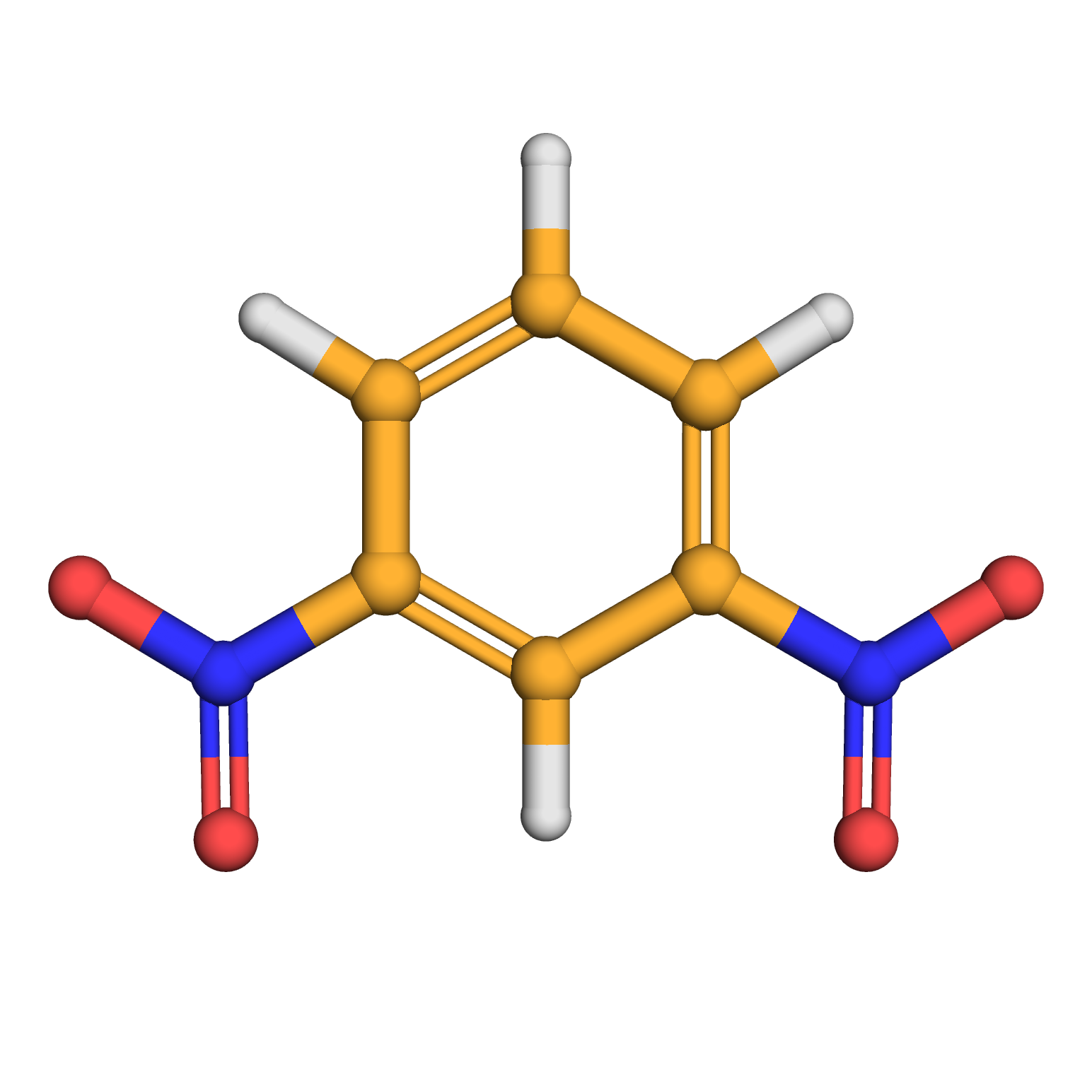
m-dinitrobenzene
Synonyms: "3-dinitrobenzene", "1,3-dinitrobenzene", "2,4-dinitrobenzene", "m-dinitrobenzene", "meta-dinitrobenzene"
Source: 1,3-dinitrobenzene is used in organic synthesis and as a camphor substitute for cellulose nitrate.
Identifiers:
IUPAC Name: 1,3-dinitrobenzene
CAS Number: 99-65-0
PubChem ID: 7452
InChiKey: WDCYWAQPCXBPJA-UHFFFAOYSA-N
Canonical SMILES: C1=CC(=CC(=C1)[N+](=O)[O-])[N+](=O)[O-]
Structural Properties:
Molecular Formula: C6H4N2O4
Molecular Weight: 168.107
Pharmacophore Features:
Number of bond donors: 0
Number of bond acceptors: 4
Number of atoms different from hydrogen: 12
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Allenby G, Foster PM, Sharpe RM. 1991. Evaluation of changes in the secretion of immunoactive inhibin by adult rat seminiferous tubules in vitro as an indicator of early toxicant action on spermatogenesis. Fundam Appl Toxicol 16(4):710-724.
Allenby G, Sharpe RM, Foster PM. 1990. Changes in Sertoli cell function in vitro induced by nitrobenzene. Fundam Appl Toxicol 14(2):364-375.
Foster PMD, Lloyd SC, Prout MS. 1987. Toxicity and metabolism of 1,3-dinitrobenzene in rat testicular cell cultures. Toxicol in Vitro 1(1):31-37.
External Links
2D-structure
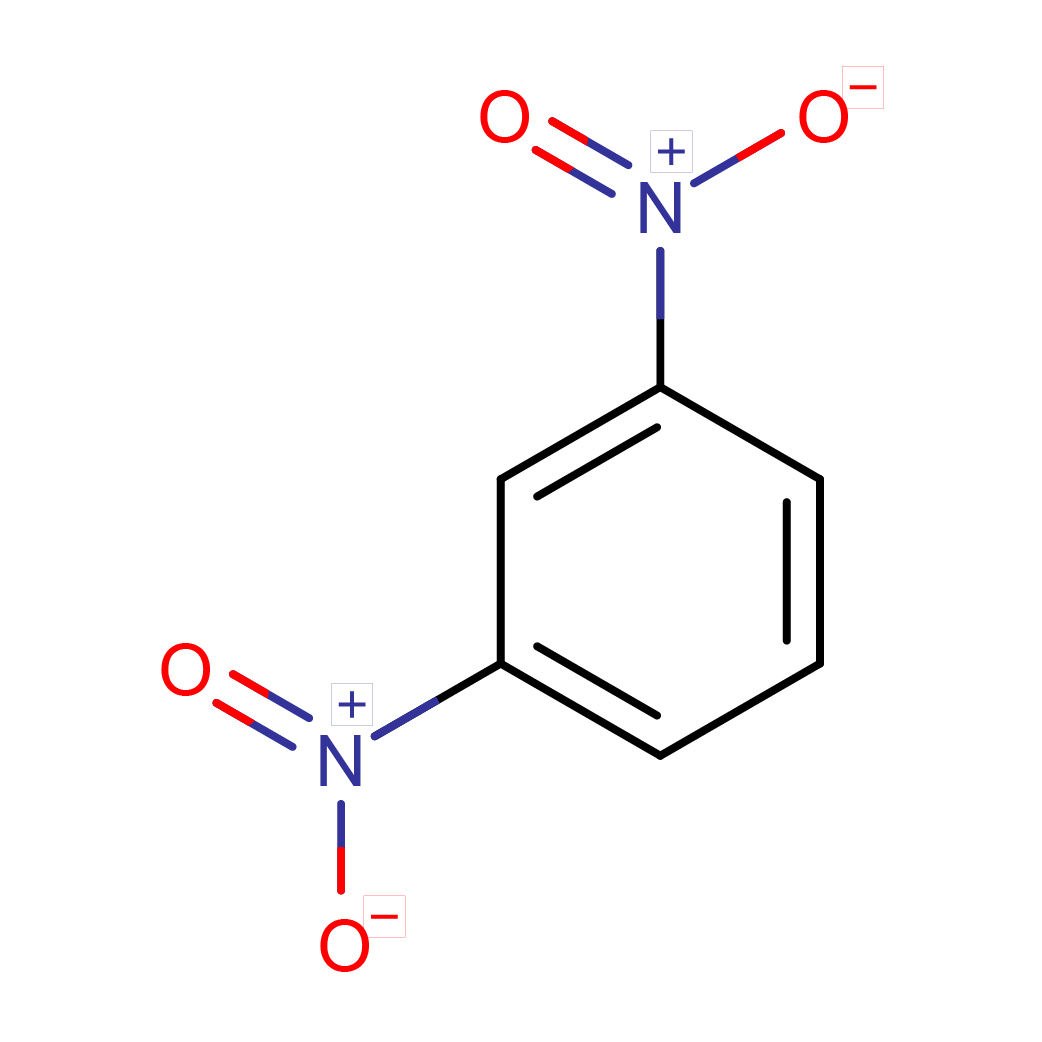
3D-structure