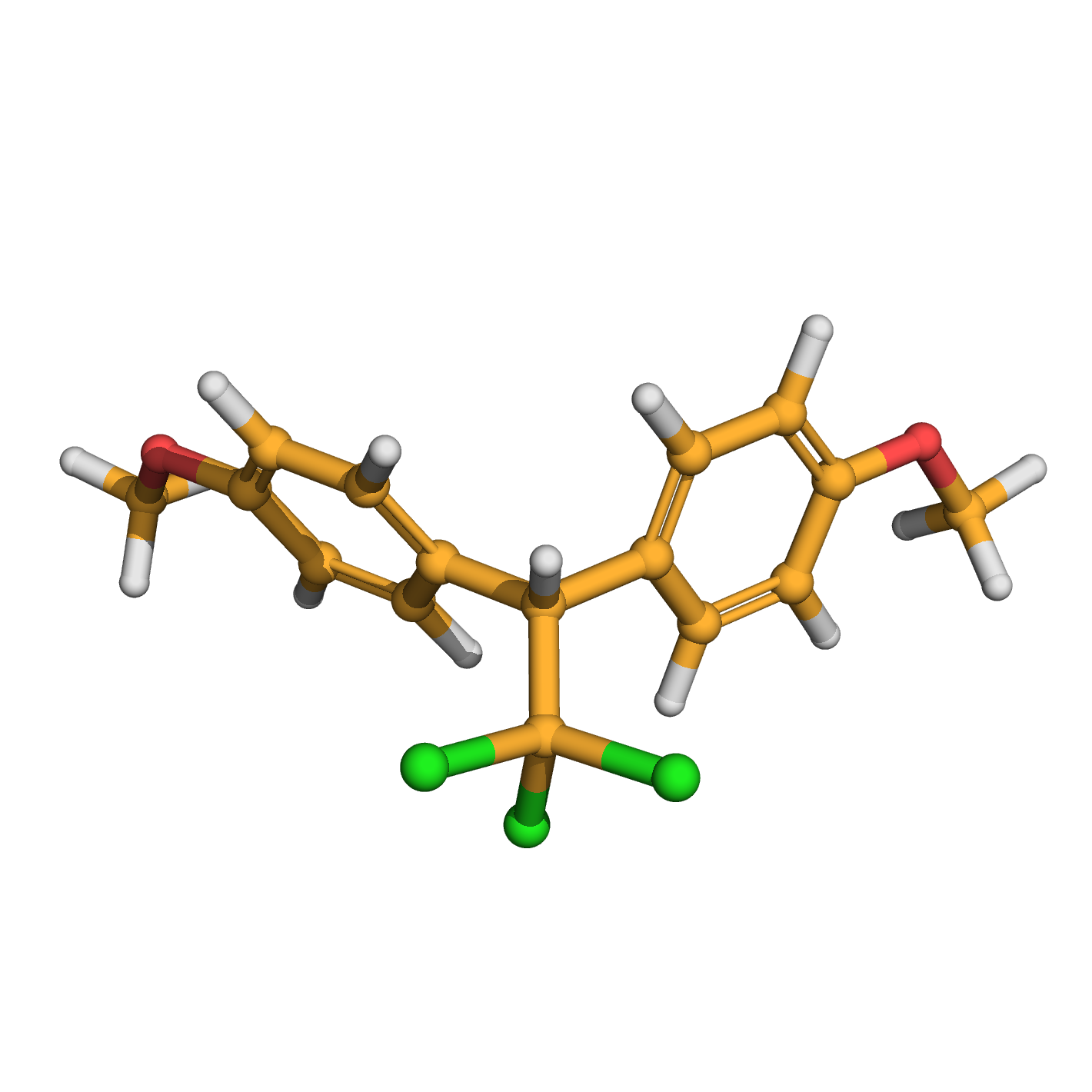
methoxychlor
Synonyms: "p,p'-methoxychlor", "DMDT", "dimethoxy-DDT", "methoxy-DDT", "methoxcide", "metox", "maralate", "marlate"
Source: methoxychlor is a synthetic organochlorine used as an insecticide.
Identifiers:
IUPAC Name: 1-methoxy-4-[2,2,2-trichloro-1-(4-methoxyphenyl)ethyl]benzene
CAS Number: 72-43-5
PubChem ID: 4115
InChiKey: IAKOZHOLGAGEJT-UHFFFAOYSA-N
Canonical SMILES: COC1=CC=C(C=C1)C(C2=CC=C(C=C2)OC)C(Cl)(Cl)Cl
Structural Properties:
Molecular Formula: C16H15Cl3O2
Molecular Weight: 345.648
Pharmacophore Features:
Number of bond donors: 0
Number of bond acceptors: 2
Number of atoms different from hydrogen: 21
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Anway MD, Cupp AS, Uzumcu M, Skinner MK. 2005. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 308(5727):1466-1469.
Danzo BJ. 1997. Environmental xenobiotics may disrupt normal endocrine function by interfering with the binding of physiological ligands to steroid receptors and binding proteins. Environ Health Perspect 105(3):294-301.
Kojima H, Katsura E, Takeuchi S, Niiyama K, Kobayashi K. 2004. Screening for estrogen and androgen receptor activities in 200 pesticides by in vitro reporter gene assays using Chinese hamster ovary cells. Environ Health Perspect 112(5):524-531.
Soto AM, Sonnenschein C, Chung KL, Fernandez MF, Olea N, Serrano FO. 1995. The E-SCREEN assay as a tool to identify estrogens: An update on estrogenic environmental pollutants. Environ Health Perspect 103 (Suppl. 7):113-122.
vom Saal FS, Nagel SC, Palanza P, Boechler M, Parmigiani S, Welshons WV. 1995. Estrogenic pesticides: Binding relative to estradiol in MCF-7 cells and effects of exposure during fetal life on subsequent territorial behavior in male mice. Toxicol Lett 77(1-3):343-350.
External Links

2D-structure
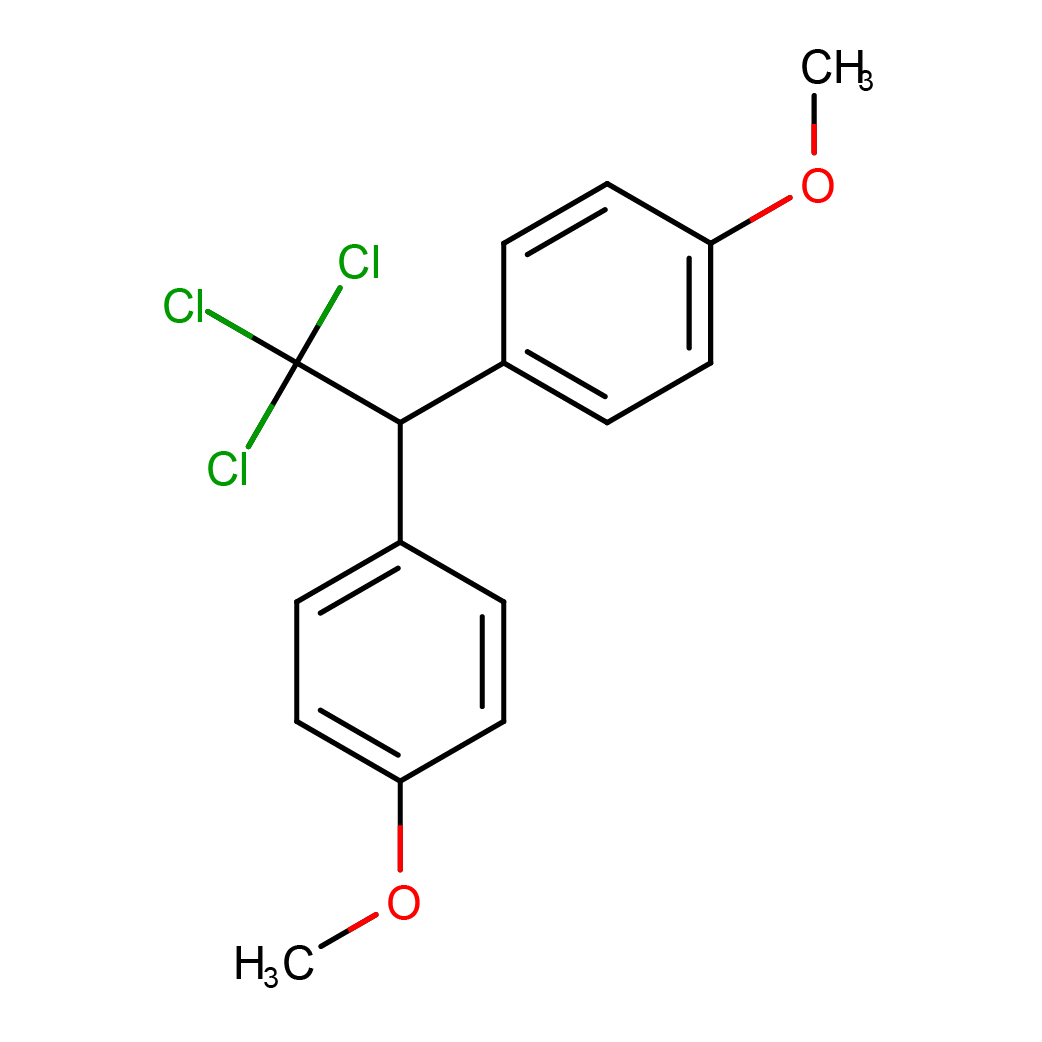
3D-structure