carbaryl
Synonyms: "carbaril", "sevin", "carylderm", "1-Naphthyl N-methylcarbamate", "caprolin", "carbatox", "carbavur", "carpolin"
Source: carbaryl is a general use carbamate pesticide that acts primarily as an insecticide, but is also competent as molluscicide and acaricide.
Identifiers:
IUPAC Name: naphthalen-1-yl N-methylcarbamate
CAS Number: 63-25-2
PubChem ID: 6129
InChiKey: CVXBEEMKQHEXEN-UHFFFAOYSA-N
Canonical SMILES: CNC(=O)OC1=CC=CC2=CC=CC=C21
Structural Properties:
Molecular Formula: C12H11NO2
Molecular Weight: 201.221
Pharmacophore Features:
Number of bond donors: 1
Number of bond acceptors: 1
Number of atoms different from hydrogen: 15
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Casale GP, Vennerstrom JL, Bavari S, Wang T. 1993. Inhibition of interleukin 2 driven proliferation of mouse CTLL2 cells, by selected carbamate and organophosphate insecticides and congeners of carbaryl. Immunopharmacology & Immunotoxicology 15(2 & 3):199-215.
Dieringer CS, Thomas JA. 1974. Effects of carbaryl on the metabolism of androgens in the prostate and liver of the mouse. Environ Res 7(3):381-386.
Hassan A. 1971. Pharmacological effects of carbaryl. I. The effect of carbaryl on the synthesis and degradation of catecholamines in the rat. Biochem Pharmacol 20(9):2299-308.
Klotz DM, Arnold SF, McLachlan JA. 1997. Inhibition of 17 beta-estradiol and progesterone activity in human breast and endometrial cancer cells by carbamate insecticides. Life Sci 60(17):1467-1475.
Smalley HE, Curtis JM, Earl FL. 1968. Teratogenic action of carbaryl in beagle dogs. Toxicol Appl Pharmacol 13(3):392-403.
External Links


2D-structure
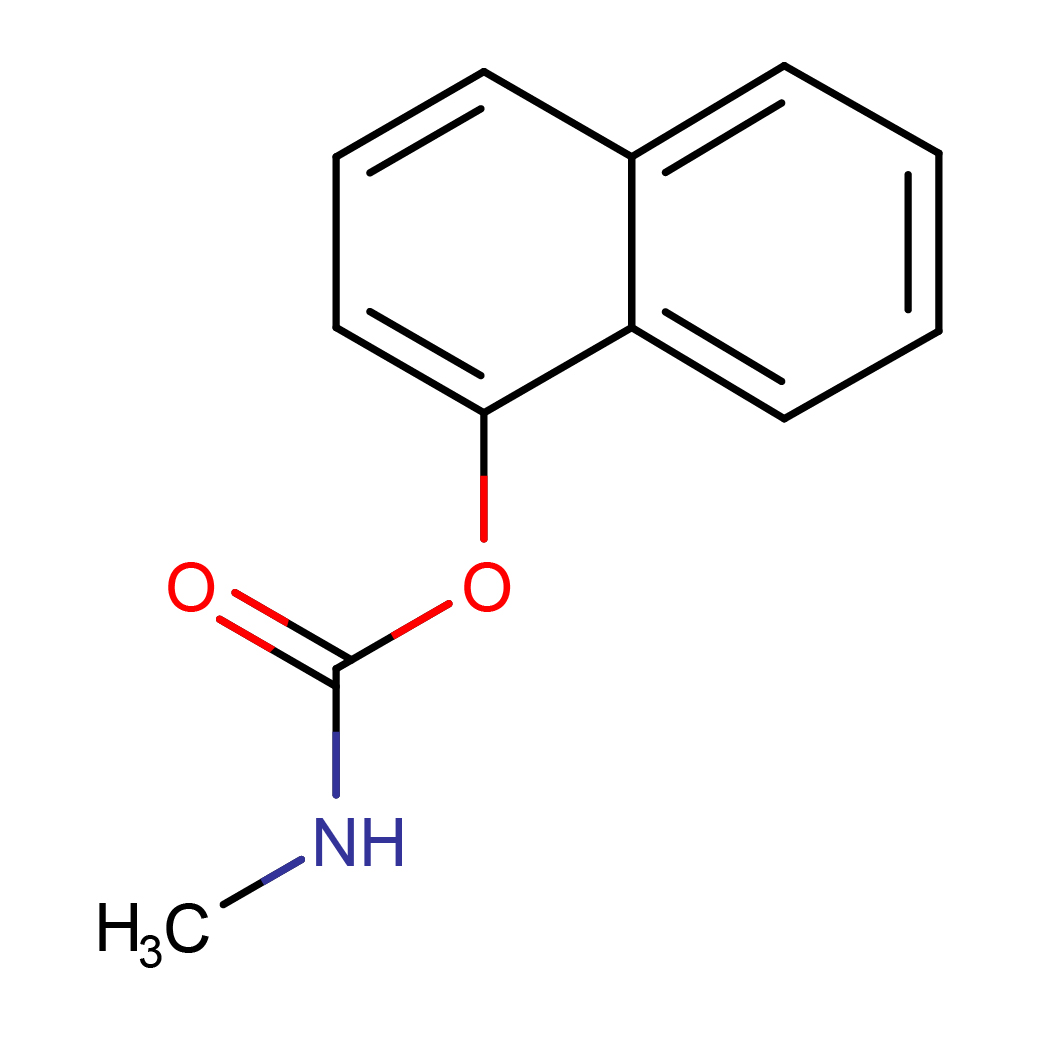
3D-structure