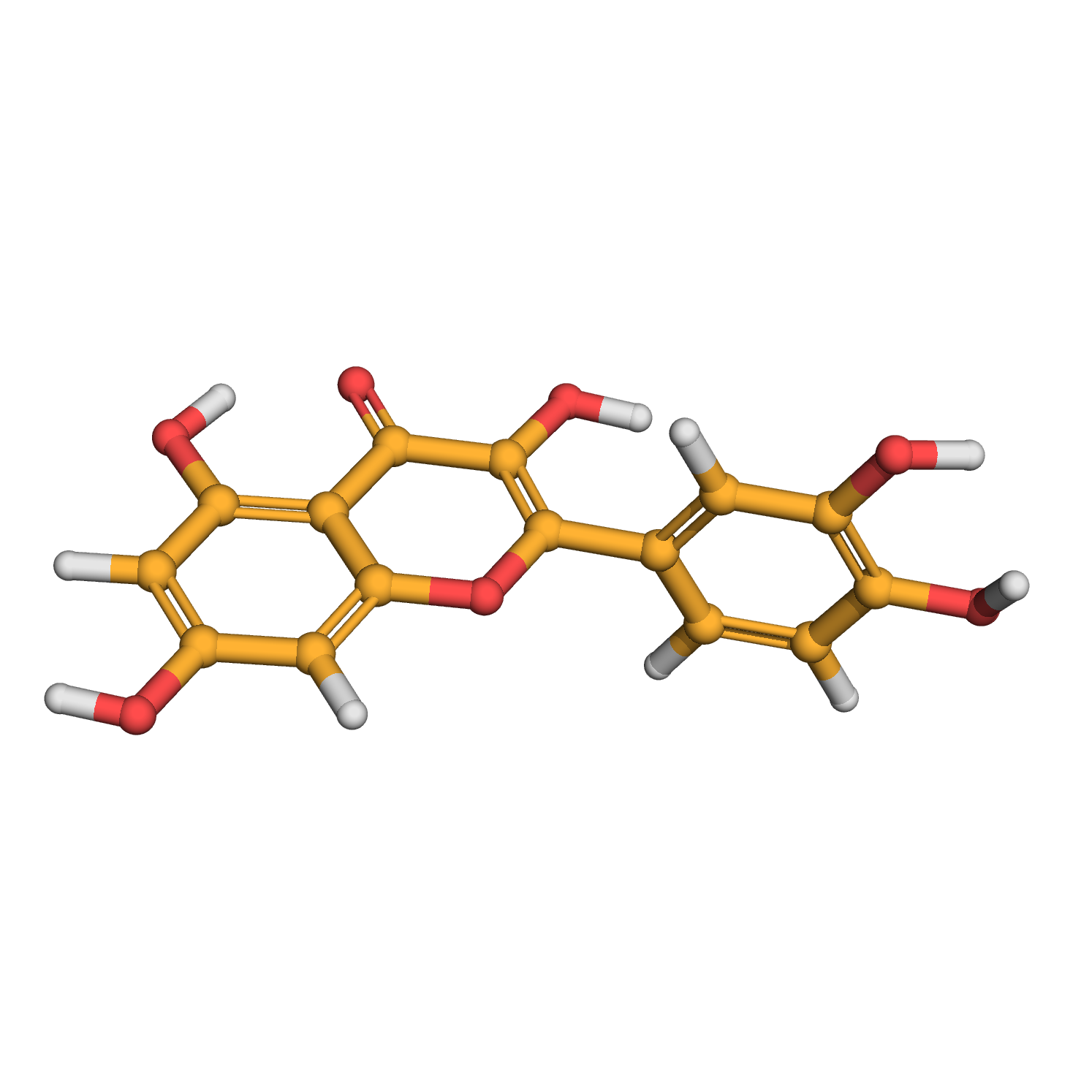
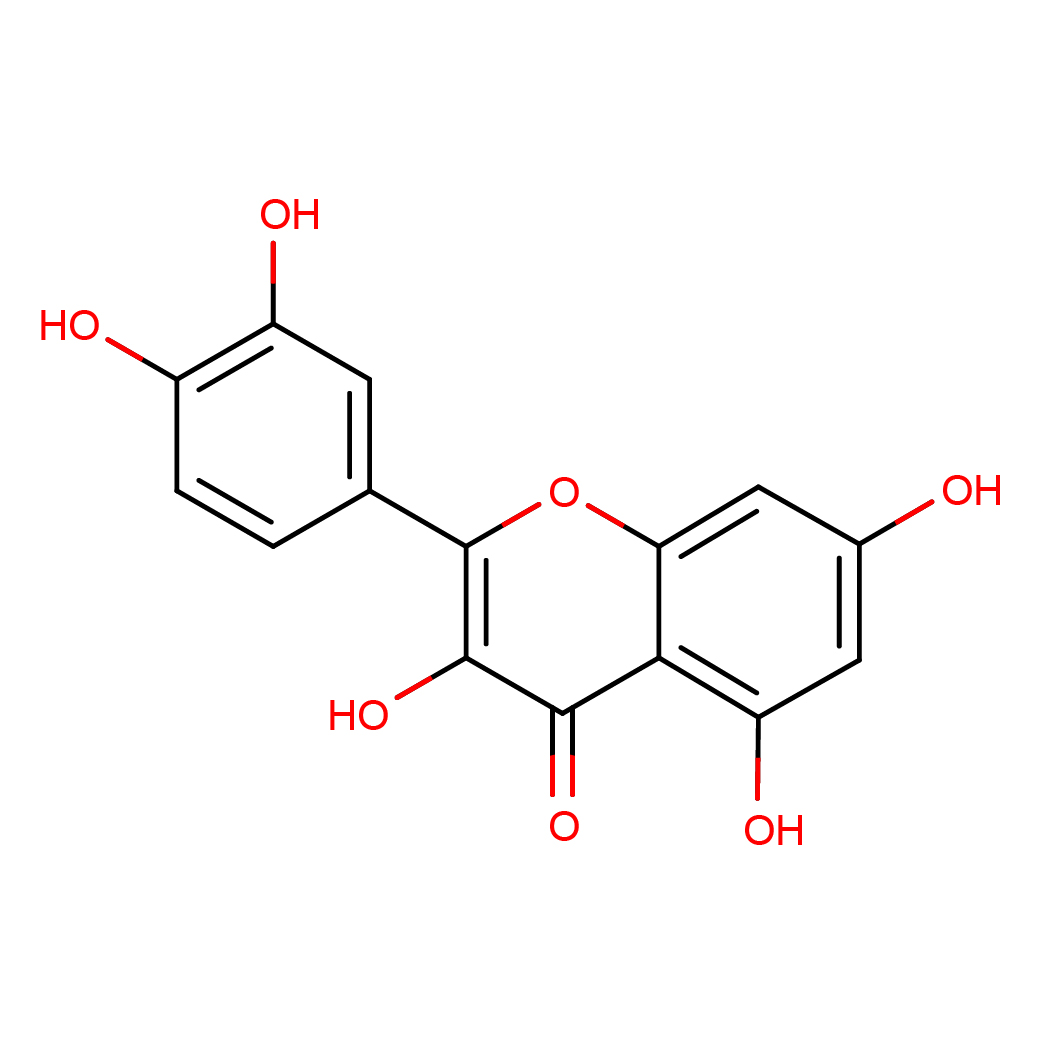
quercetin
Synonyms: "sophoretin", "meletin", "xanthaurine", "quercetine", "quercetol", "quercitin", "quertine", "flavin meletin"
Source: quercetin is a flavonoid widely distributed in nature.
Identifiers:
IUPAC Name: 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxychromen-4-one
CAS Number: 117-39-5
PubChem ID: 5280343
InChiKey: REFJWTPEDVJJIY-UHFFFAOYSA-N
Canonical SMILES: C1=CC(=C(C=C1C2=C(C(=O)C3=C(C=C(C=C3O2)O)O)O)O)O
Structural Properties:
Molecular Formula: C15H10O7
Molecular Weight: 302.236
Pharmacophore Features:
Number of bond donors: 5
Number of bond acceptors: 7
Number of atoms different from hydrogen: 22
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Kuiper GGJM, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg P, Gustafsson JA. 1998. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 139(10):4252-4263.Kuiper GGJM, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg P, Gustafsson JA. 1998. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 139(10):4252-4263.Oberdö,rster E, Clay MA, Cottam DM, Wilmot FA, McLachlan JA, Milner MJ. 2001. Common phytochemicals are ecdysteroid agonists and antagonists: a possible evolutionary link between vertebrate and invertebrate steroid hormones. J Steroid Biochem Mol Biol 77(4-5):229-238, DOI: 10.1016/S0960-0760(01)00067-X.Oberdö,rster E, Clay MA, Cottam DM, Wilmot FA, McLachlan JA, Milner MJ. 2001. Common phytochemicals are ecdysteroid agonists and antagonists: a possible evolutionary link between vertebrate and invertebrate steroid hormones. J Steroid Biochem Mol Biol 77(4-5):229-238, DOI: 10.1016/S0960-0760(01)00067-X.
External Links
2D-structure
3D-structure