4-nonylphenol
Synonyms: "p-nonylphenol", "para-nonylphenol", "4-n-nonylphenol", "p-n-nonylphenol"
Source: 4-nonylphenol is commonly used as a raw material for making detergents, pesticides, anti-oxidants in plastics and rubbers, as a supplemental agent/stabilizer of polyvinyl chloride, as well as other uses.
Identifiers:
IUPAC Name: 4-nonylphenol
CAS Number: 104-40-5
PubChem ID: 1752
InChiKey: IGFHQQFPSIBGKE-UHFFFAOYSA-N
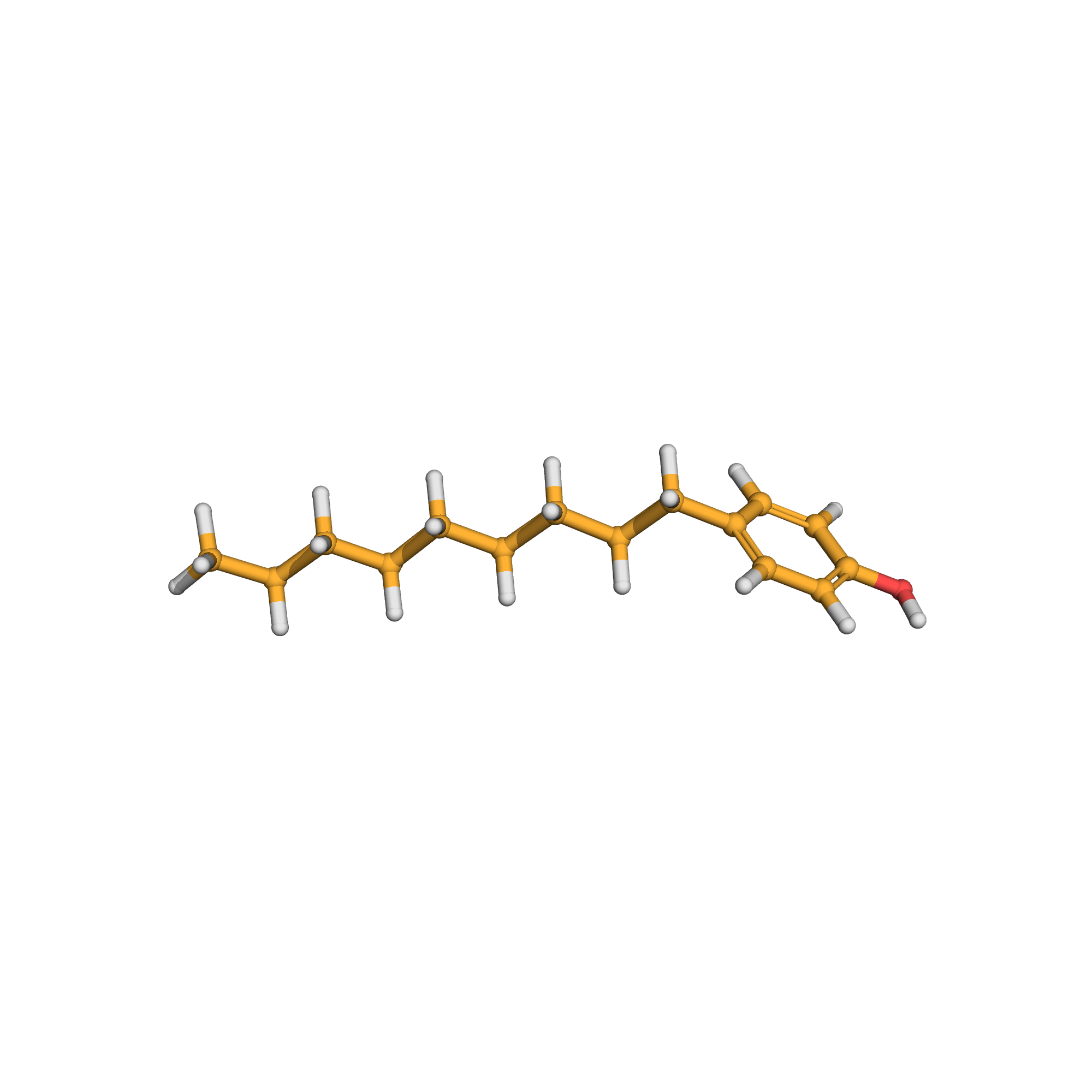
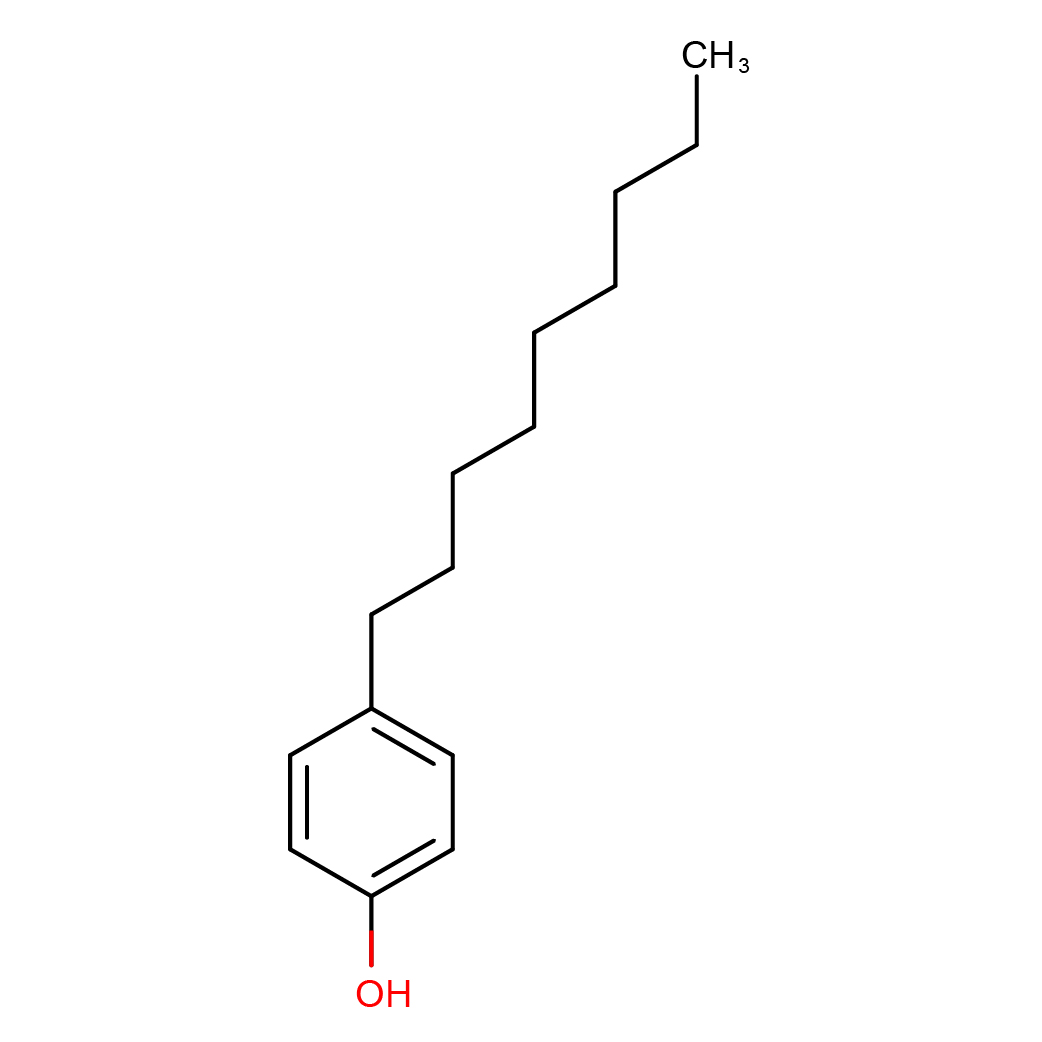
Canonical SMILES: CCCCCCCCCC1=CC=C(C=C1)O
Structural Properties:
Molecular Formula: C15H24O
Molecular Weight: 220.351
Pharmacophore Features:
Number of bond donors: 1
Number of bond acceptors: 1
Number of atoms different from hydrogen: 16
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Jobling S, Sheahan D, Osborne JA, Matthiessen P, Sumpter JP. 1996. Inhibition of testicular growth in rainbow trout (Oncorhynchus mykiss) exposed to estrogenic alkylphenolic chemicals. Environ Toxicol Chem 15(2):194-202.
Kanayama T, Kobayashi N, Mamiya S, Nakanishi T, Nishikawa J. 2005. Organotin compounds promote adipocyte differentiation as agonists of the peroxisome proliferator-activated receptor [gamma]/retinoid X receptor pathway. Mol Pharmacol 67(3):766-774.
Matsunaga H, Mizota K, Uchida H, Uchida T, Ueda H. 2010. Endocrine disrupting chemicals bind to a novel receptor, microtubule-associated protein 2, and positively and negatively regulate dendritic outgrowth in hippocampal neurons. J Neurochem 114(5):1333-1343.
Soto AM, Justicia H, Wray JW, Sonnenschein C. 1991. p-Nonyl-phenol: An estrogenic xenobiotic released from "modified" polystyrene. Environ Health Perspect 92:167-173.Tran DQ, Klotz DM, Ladlie BL, Ide CF, McLachlan JA, Arnold SF. 1996. Inhibition of progesterone receptor activity in yeast by synthetic chemicals. Biochem Biophys Res Commun 229(2):518-523.
External Links



2D-structure
3D-structure