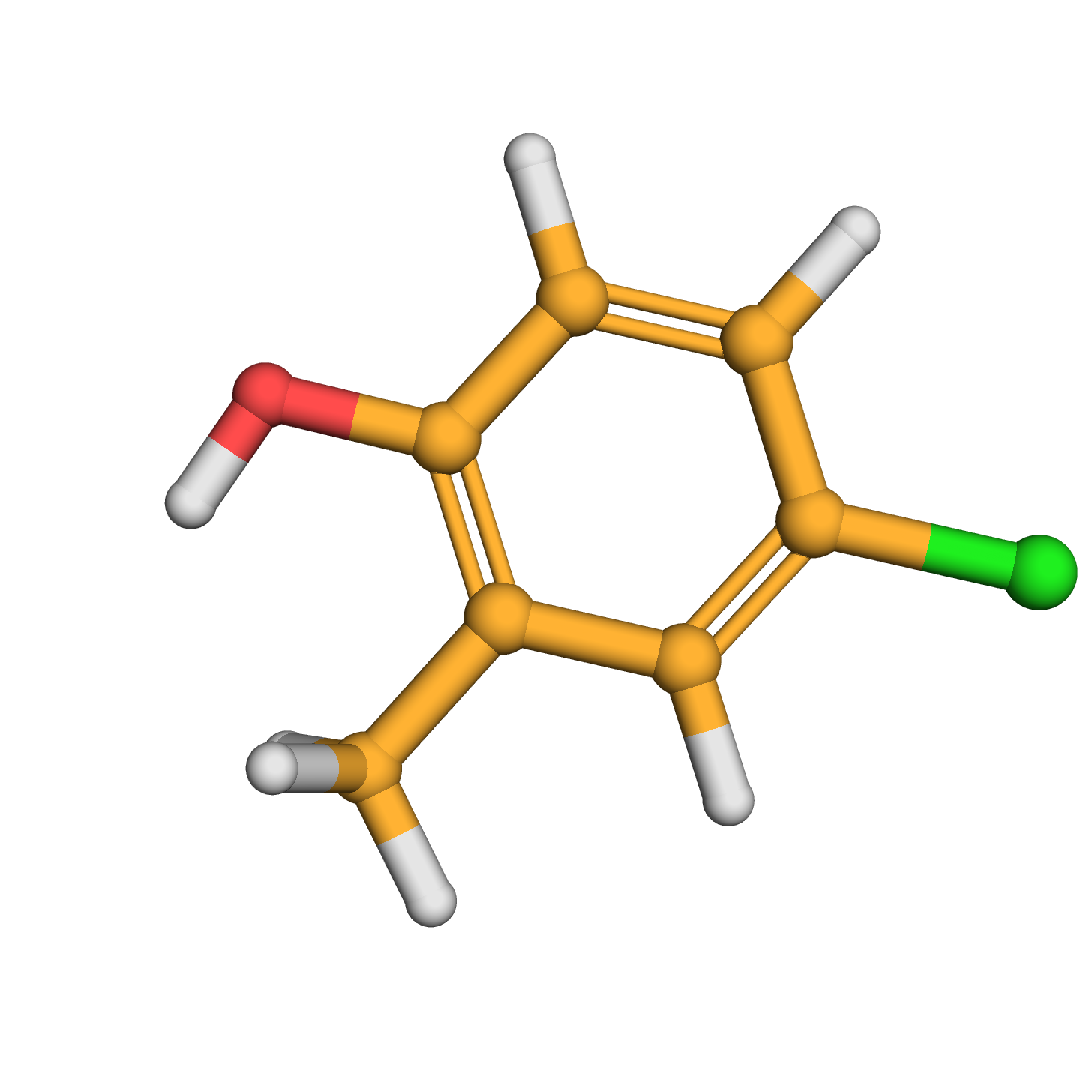
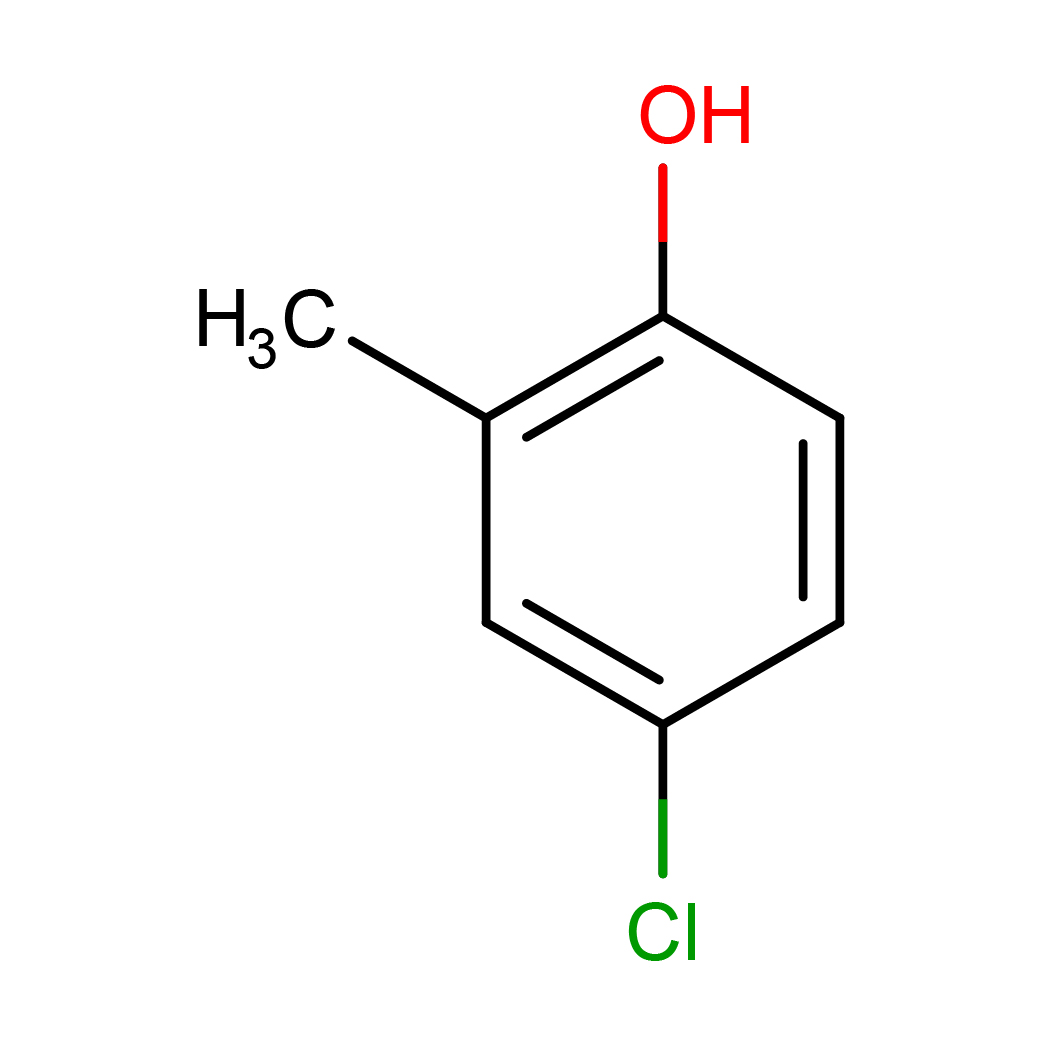
4-chloro-2-methylphenol
Synonyms: "4-chloro-2-cresol", "4-chloro-o-cresol", "p-chloro-o-cresol"
Source: 4-chloro-2-methylphenol is used as chemical intermediate in the synthesis of chlorophenoxyacetic acid herbicides, e.g. MCPA, MCPB, and mecoprop.
Identifiers:
IUPAC Name: 4-chloro-2-methylphenol
CAS Number: 1570-64-5
PubChem ID: 14855
InChiKey: RHPUJHQBPORFGV-UHFFFAOYSA-N
Canonical SMILES: CC1=C(C=CC(=C1)Cl)O
Structural Properties:
Molecular Formula: C7H7ClO
Molecular Weight: 142.583
Pharmacophore Features:
Number of bond donors: 1
Number of bond acceptors: 1
Number of atoms different from hydrogen: 9
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Korner W, Hanf V, Schuller W, Bartsch H, Zwirner M, Hagenmaier H. 1998. Validation and application of a rapid in vitro assay for assessing the estrogenic potency of halogenated phenolic chemicals. Chemosphere 37(9-12):2395-2407.
Vismara C, Bernardini G, Bordone L, Spinelli O, Teruzzi A, Rossetti C. 1995. Effects of chlorocresol (4-chloro-2-methyl phenol) administered during the fertilization and cleavage phases of Xenopus laevis. Bull Environ Contam Toxicol 55(2):195-200.
External Links


2D-structure
3D-structure