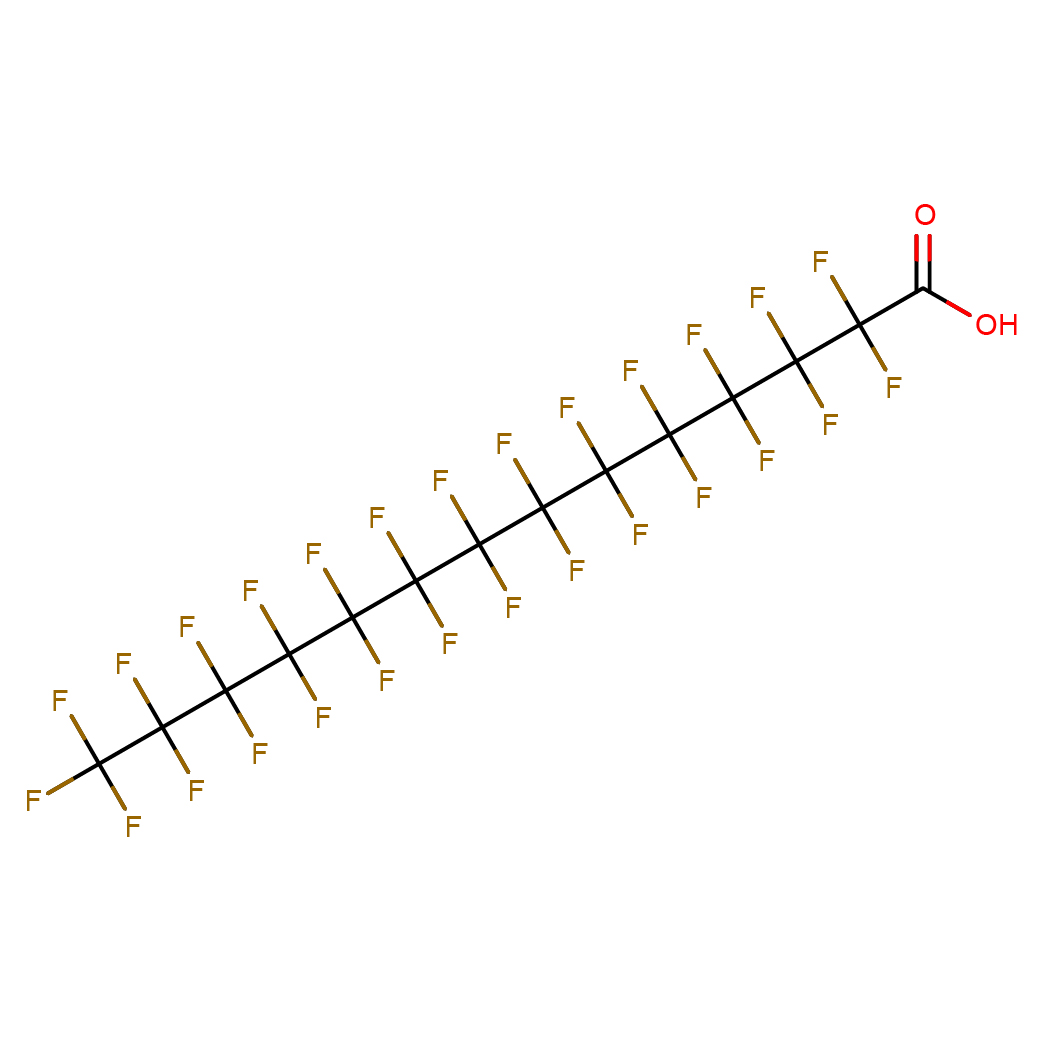
Perfluorotetradecanoic acid
Synonyms: "perfluoromyristic acid", "Heptacosafluorotetradecanoic acid", "2,2,3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,12,12,13,13,14,14,14-heptacosafluorotetradecanoic acid".
Source: Perfluorotetradecanoic acid is used for GC (Gas Chromatography) and LC (Liquid Chromatography) analysis.
Identifiers:
IUPAC Name: 2,2,3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,12,12,13,13,14,14,14-heptacosafluorotetradecanoic acid
CAS Number: 376-06-7
PubChem ID: 67822
InChiKey: RUDINRUXCKIXAJ-UHFFFAOYSA-N
Canonical SMILES: C(=O)(C(C(C(C(C(C(C(C(C(C(C(C(C(F)(F)F)(F)F)(F)F)(F)F)(F)F)(F)F)(F)F)(F)F)(F)F)(F)F)(F)F)(F)F)(F)F)O
Structural Properties:
Molecular Formula: C14HF27O2
Molecular Weight: 714.117
Pharmacophore Features:
Number of bond donors: 1
Number of bond acceptors: 29
Number of atoms different from hydrogen: 43
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Ren XM, Qin WP, Cao LY, Zhang J, Yang Y, Wan B and Guo LH. 2016. Binding interactions of perfluoroalkyl substances with thyroid hormone transport proteins and potential toxicological implications. Toxicology 366:32-42. DOI: 10.1016/j.tox.2016.08.011. URL: https://www.ncbi.nlm.nih.gov/pubmed/27528273.
Rosenmai AK, Ahrens L, Godec T, Lundqvist J and Oskarsson A. 2017. Relationship between peroxisome proliferator?activated receptor alpha activity and cellular concentration of 14 perfluoroalkyl substances in HepG2 cells. J Appl Toxicol 38(2):219-226. DOI: 10.1002/jat.3515. URL: https://www.ncbi.nlm.nih.gov/pubmed/28857218.
External Links

2D-structure
3D-structure