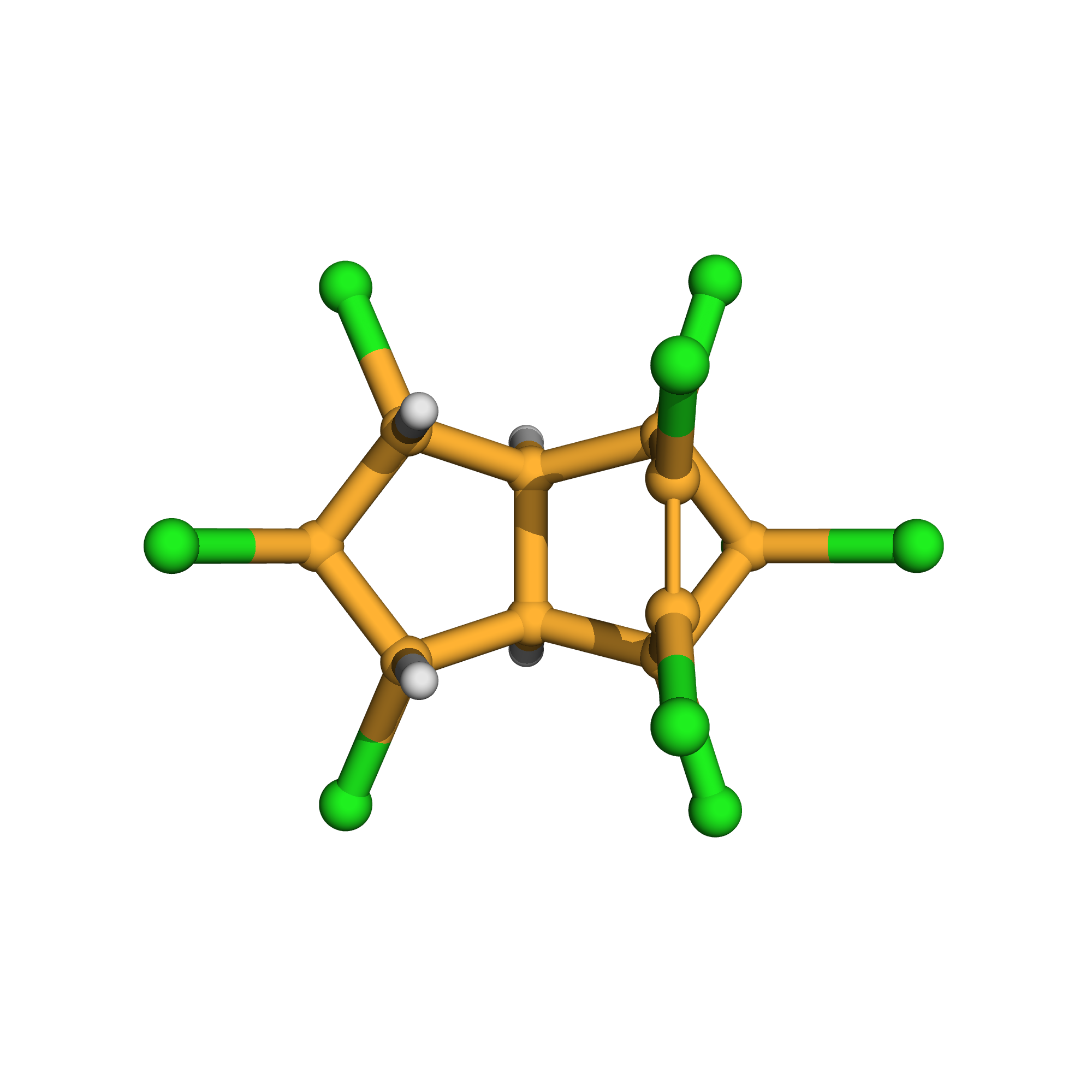
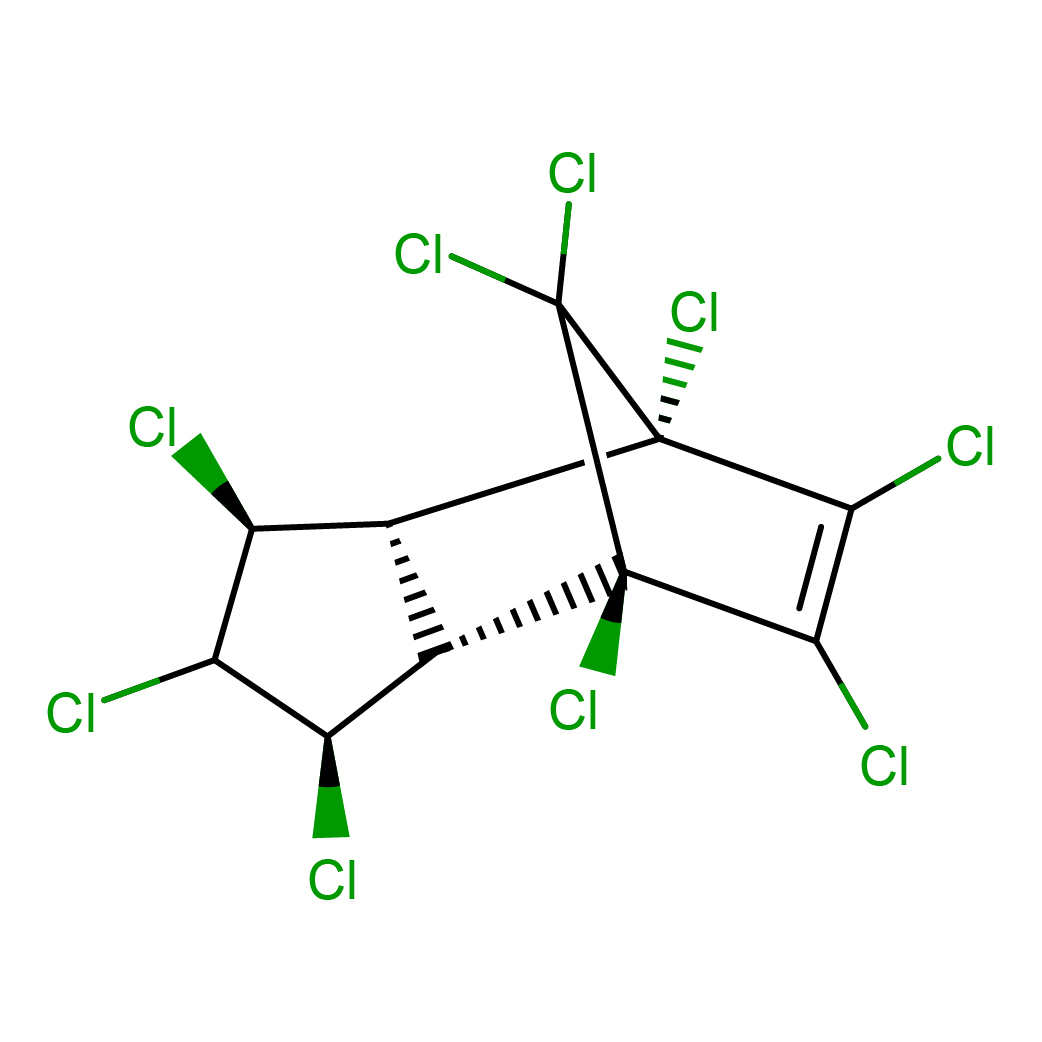
trans-nonachlor
Synonyms: "t-nonachlor".
Source: Trans-nonachlor is a major component of the organochlorine pesticide chlordane.
Identifiers:
IUPAC Name: (1S,2R,3S,5R,6S,7R)-1,3,4,5,7,8,9,10,10-nonachlorotricyclo[5.2.1.02,6]dec-8-ene
CAS Number: 5103-73-1
PubChem ID: 12313421
InChiKey: OCHOKXCPKDPNQU-FLVMBEMLSA-N
Canonical SMILES: C12C(C(C(C1Cl)Cl)Cl)C3(C(=C(C2(C3(Cl)Cl)Cl)Cl)Cl)Cl
Structural Properties:
Molecular Formula: C10H5Cl9
Molecular Weight: 444.200
Pharmacophore Features:
Number of bond donors: 0
Number of bond acceptors: 0
Number of atoms different from hydrogen: 19
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Klotz DM, Beckman BS, Hill SM, McLachlan JA, Walters MR, Arnold SF. 1996. Identification of environmental chemicals with estrogenic activity using a combination of in vitro assays. Environ Health Perspect 104(10):1084-1089. DOI: 10.2307/3433121 . URL: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1469483/.
Scippo ML, Argiris C, Van De Weerdt C, Muller M, Willemsen P, Martial J, Maghuin-Rogister G. 2004. Recombinant human estrogen, androgen and progesterone receptors for detection of potential endocrine disruptors. Anal Bioanal Chem 378(3):664-669. DOI: 10.1007/s00216-003-2251-0. URL: https://link.springer.com/article/10.1007/s00216-003-2251-0.
Vonier PM, Crain DA, McLachlan JA, Guillette LJ Jr., Arnold SF. 1996. Interaction of environmental chemicals with the estrogen and progesterone receptors from the oviduct of the American alligator. Environ Health Perspect 104(12):1318-1322. DOI: 10.2307/3432968 . URL: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1469547/.
External Links

2D-structure
3D-structure