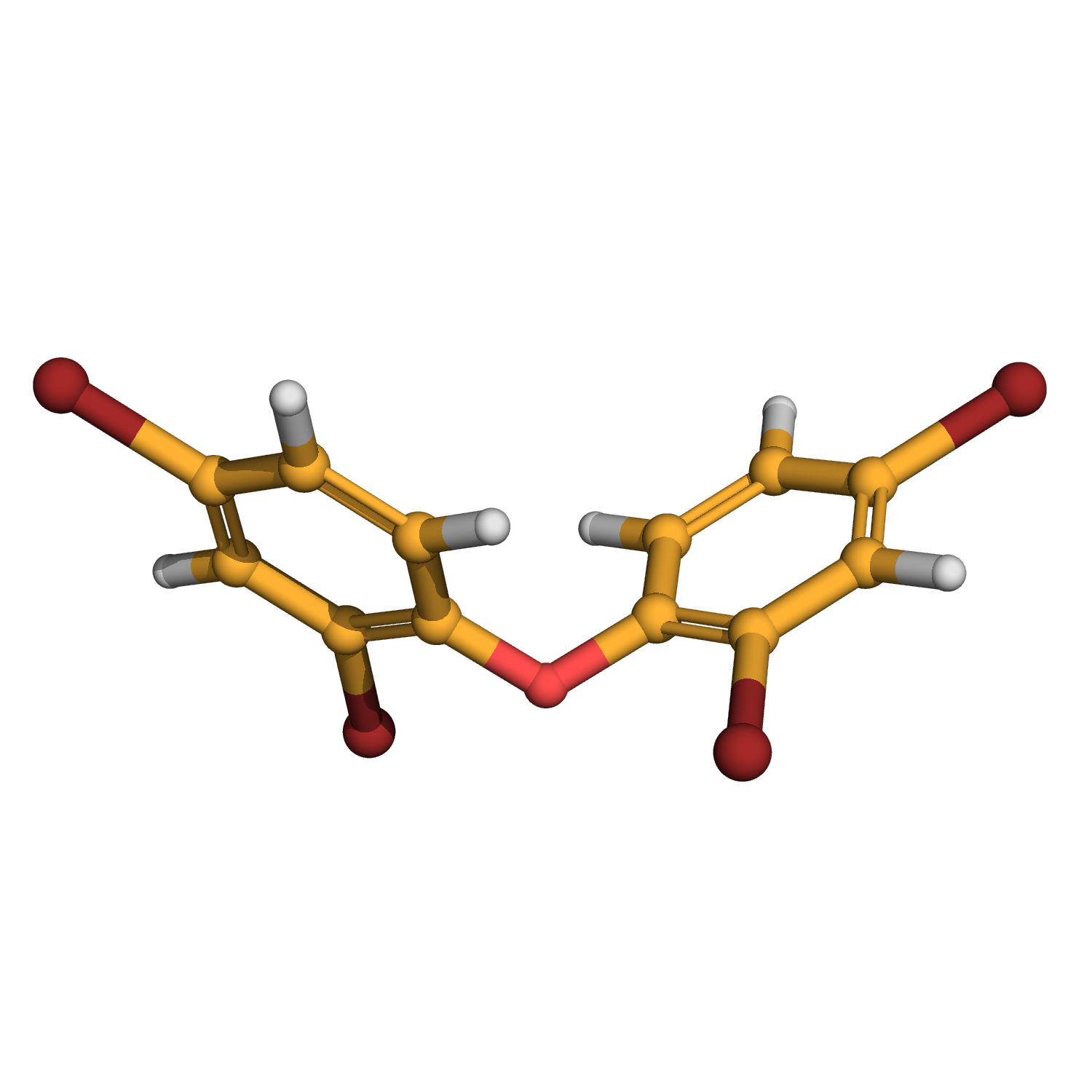
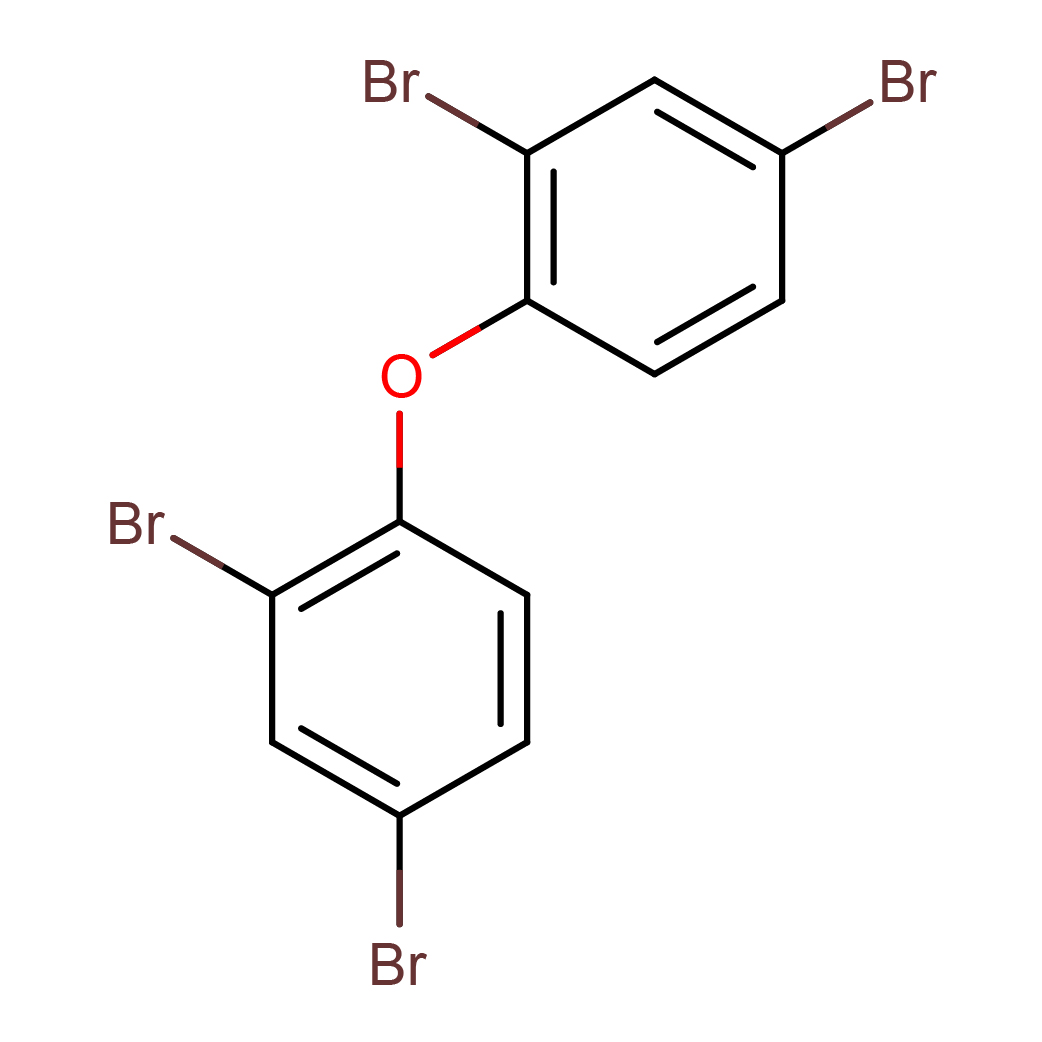
2,2',4,4'-tetrabromodiphenyl ether
Synonyms: "PBDE 47", "dibromophenyl ether", "bis(2,4-dibromophenyl) ether"
Source: 2,2',4,4'-tetrabromodiphenyl ether is the major PBDE (Polybrominated diphenyl ethers) congener detected in the environment and in animal tissues.
Identifiers:
IUPAC Name: 2,4-dibromo-1-(2,4-dibromophenoxy)benzene
CAS Number: 5436-43-1
PubChem ID: 95170
InChiKey: XYBSIYMGXVUVGY-UHFFFAOYSA-N
Canonical SMILES: C1=CC(=C(C=C1Br)Br)OC2=C(C=C(C=C2)Br)Br
Structural Properties:
Molecular Formula: C12H6Br4O
Molecular Weight: 485.791
Pharmacophore Features:
Number of bond donors: 0
Number of bond acceptors: 0
Number of atoms different from hydrogen: 17
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Hamers T, Kamstra JH, Sonneveld E, Murk AJ, Kester MH, Andersson PL, Legler J, Brouwer A. 2006. In vitro profiling of the endocrine-disrupting potency of brominated flame retardants. Toxicol Sci 92(1):157-173, doi: 10.1093/toxsci/kfj187.
Hamers T, Kamstra JH, Sonneveld E, Murk AJ, Visser TJ, Van Velzen MJM, Brouwer A, Bergman Å,. 2008. Biotransformation of brominated flame retardants into potentially endocrine-disrupting metabolites, with special attention to 2,2',4,4'-tetrabromodiphenyl ether (BDE-47). Mol Nutr Food Res 52(2):284-298, doi: 10.1002/mnfr.200700104.
Meerts IATM, Letcher RJ, Hoving S, Marsh G, Bergman Å,, Lemmen JG, van der Burg B, Brouwer A. 2001. In vitro estrogenicity of polybrominated diphenyl ethers, hydroxylated PBDEs, and polybrominated bisphenol A compounds. Environ Health Perspect 109(4):399-407, DOI: 10.2307/3454900.
External Links

2D-structure
3D-structure