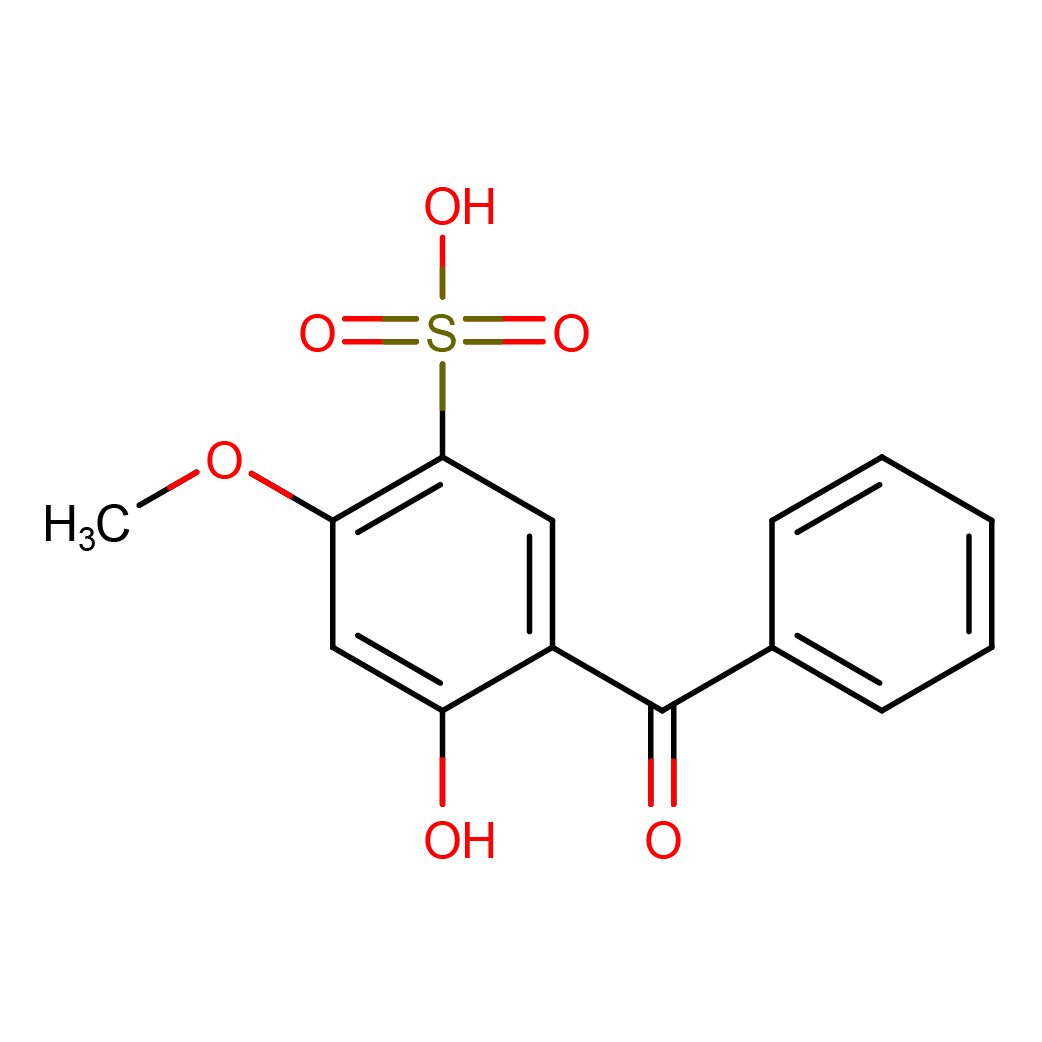
sulisobenzone
Synonyms: "5-benzoyl-4-hydroxy-2-methoxybenzenesulfonic acid", "2-hydroxy-4-methoxybenzophenone-5-sulfonic acid", "2-hydroxy-4-methoxy-5-sulfobenzophenone", "sungard", "benzophenone 4", "Uval", "Sulisobenzona", "Sulisobenzonum", "Uvinul", "Seesorb 101S", "Syntase 230", "Uvinul MS 40", "sulisobenzone", "benzophenone-4", "MS 40".
Source: Sulisobenzone is widely employed in sunscreens and other personal care products.
Identifiers:
IUPAC Name: 5-benzoyl-4-hydroxy-2-methoxybenzenesulfonic acid
CAS Number: 4065-45-6
PubChem ID: 19988
InChiKey: CXVGEDCSTKKODG-UHFFFAOYSA-N
Canonical SMILES: COC1=C(C=C(C(=C1)O)C(=O)C2=CC=CC=C2)S(=O)(=O)O
Structural Properties:
Molecular Formula: C14H12O6S
Molecular Weight: 308.304
Pharmacophore Features:
Number of bond donors: 2
Number of bond acceptors: 6
Number of atoms different from hydrogen: 21
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Kunz PY, Fent K. 2006. Multiple hormonal activities of UV filters and comparison of in vivo and in vitro estrogenic activity of ethyl-4-aminobenzoate in fish. Aquat Toxicol 79(4):305-324. DOI: 10.1016/j.aquatox.2006.06.016. URL: https://www.sciencedirect.com/science/article/pii/S0166445X06002700Kunz PY, Galicia HF, Fent K. 2006. Comparison of in vitro and in vivo estrogenic activity of UV filters in fish. Toxicol Sci 90(2):349-361 DOI: 10.1093/toxsci/kfj082. URL: https://academic.oup.com/toxsci/article/90/2/349/1658390.
External Links


2D-structure
3D-structure