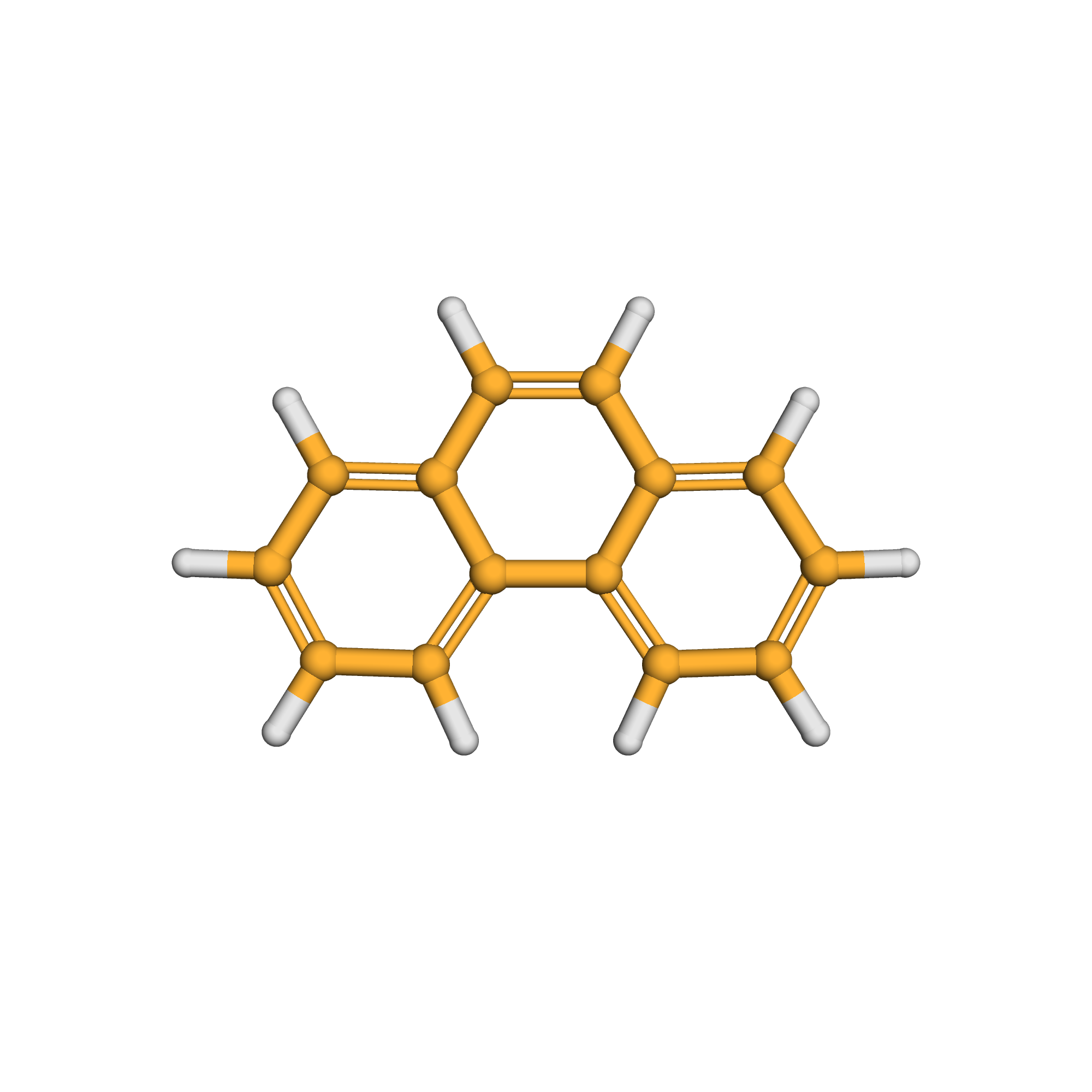
phenanthrene
Synonyms: "phenanthren", "phenanthrin", "phenanthracene", "ravatite", "phenantrin".
Source: Phenanthrene is produced by incomplete combustion of fossil fuels, and from plant-derived precursors in sediments. Comercially, phenanthrene is used to make dyes, plastics, pesticides, explosives, drugs and steroids
Identifiers:
IUPAC Name: phenanthrene
CAS Number: 85-01-8
PubChem ID: 995
InChiKey: YNPNZTXNASCQKK-UHFFFAOYSA-N
Canonical SMILES: C1=CC=C2C(=C1)C=CC3=CC=CC=C32
Structural Properties:
Molecular Formula: C14H10
Molecular Weight: 178.234
Pharmacophore Features:
Number of bond donors: 0
Number of bond acceptors: 0
Number of atoms different from hydrogen: 14
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Benisek M, Kubincova P, Blaha L, Hilscherova K. 2011. The effects of PAHs and N-PAHs on retinoid signaling and Oct-4 expression in vitro. Toxicol Lett 200(3):169-175. DOI: 10.1016/j.toxlet.2010.11.011. URL: http://www.sciencedirect.com/science/article/pii/S0378427410017716?via%3Dihub.
Evans AD, Nipper M. 2007. Toxicity of phenanthrene and lindane mixtures to marine invertebrates. Environmental Toxicology 22(5):495-501. DOI: 10.1002/tox.20279. URL: http://onlinelibrary.wiley.com/doi/10.1002/tox.20279/abstractIncardona JP, Collier TK, Scholz NL. 2004. Defects in cardiac function precede morphological abnormalities in fish embryos exposed to polycyclic aromatic hydrocarbons. Toxicol Appl Pharmacol 196(2):191-205. DOI: 10.1016/j.taap.2003.11.026. URL: https://www.sciencedirect.com/science/article/pii/S0041008X04000110?via%3Dihub.
Monteiro PRR, Reis-Henriques MA, Coimbra J. 2000. Plasma steroid levels in female flounder (Platichthys flesus) after chronic dietary exposure to single polycyclic aromatic hydrocarbons. Marine Environmental Research 49(5):453-467. DOI: 10.1016/S0141-1136(99)00085-9. URL: https://www.sciencedirect.com/science/article/pii/S0141113699000859.
Monteiro PRR, Reis-Henriques MA, Coimbra J. 2000. Polycyclic aromatic hydrocarbons inhibit in vitro ovarian steroidogenesis in the flounder (Platichthys flesus L.). Aquatic Toxicology 48(4):549-559. DOI: 10.1016/S0166-445X(99)00055-7. URL: https://www.sciencedirect.com/science/article/pii/S0166445X99000557.
External Links



2D-structure
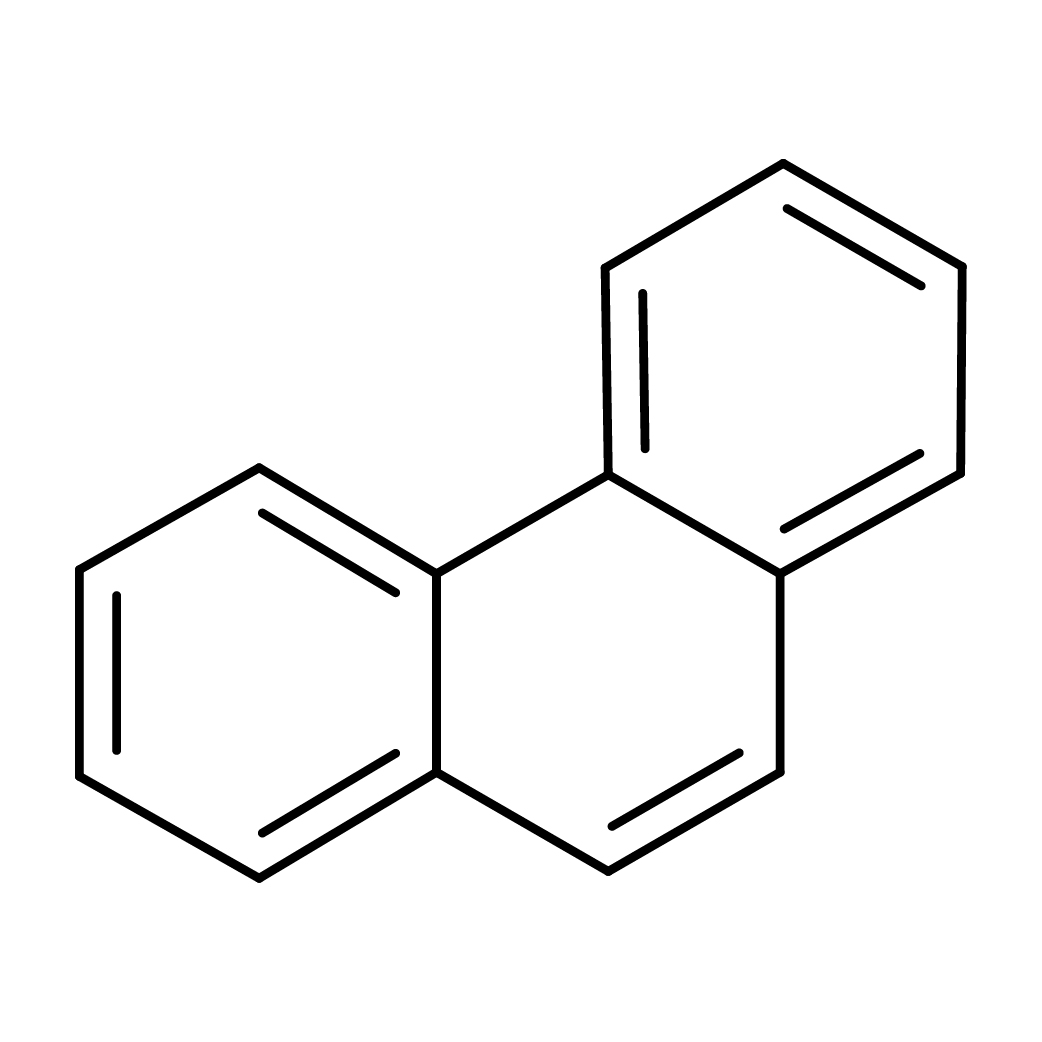
3D-structure