kojic acid
Synonyms: "5-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one", "5-hydroxy-2-(hydroxymethyl)-4-pyrone", "5-hydroxy-2-hydroxymethyl-4-pyrone", "5-hydroxy-2-(hydroxymethyl)pyran-4-one", "2-(Hydroxymethyl)-5-hydroxy-4H-pyran-4-one", "5-hydroxy-2-hydroxymethyl-4H-4-pyranone", "pyran-4-one, 5-hydroxy-2-(hydroxymethyl)", "5-Hydroxy-2-hydroxymethyl-4H-pyran-4-on"
Source: Kojic acid is used as a key ingredient in the preparation of skin care products because it acts as a skin lightening and depigmenting agent
Identifiers:
IUPAC Name: 5-hydroxy-2-(hydroxymethyl)pyran-4-one
CAS Number: 501-30-4
PubChem ID: 3840
InChiKey: BEJNERDRQOWKJM-UHFFFAOYSA-N
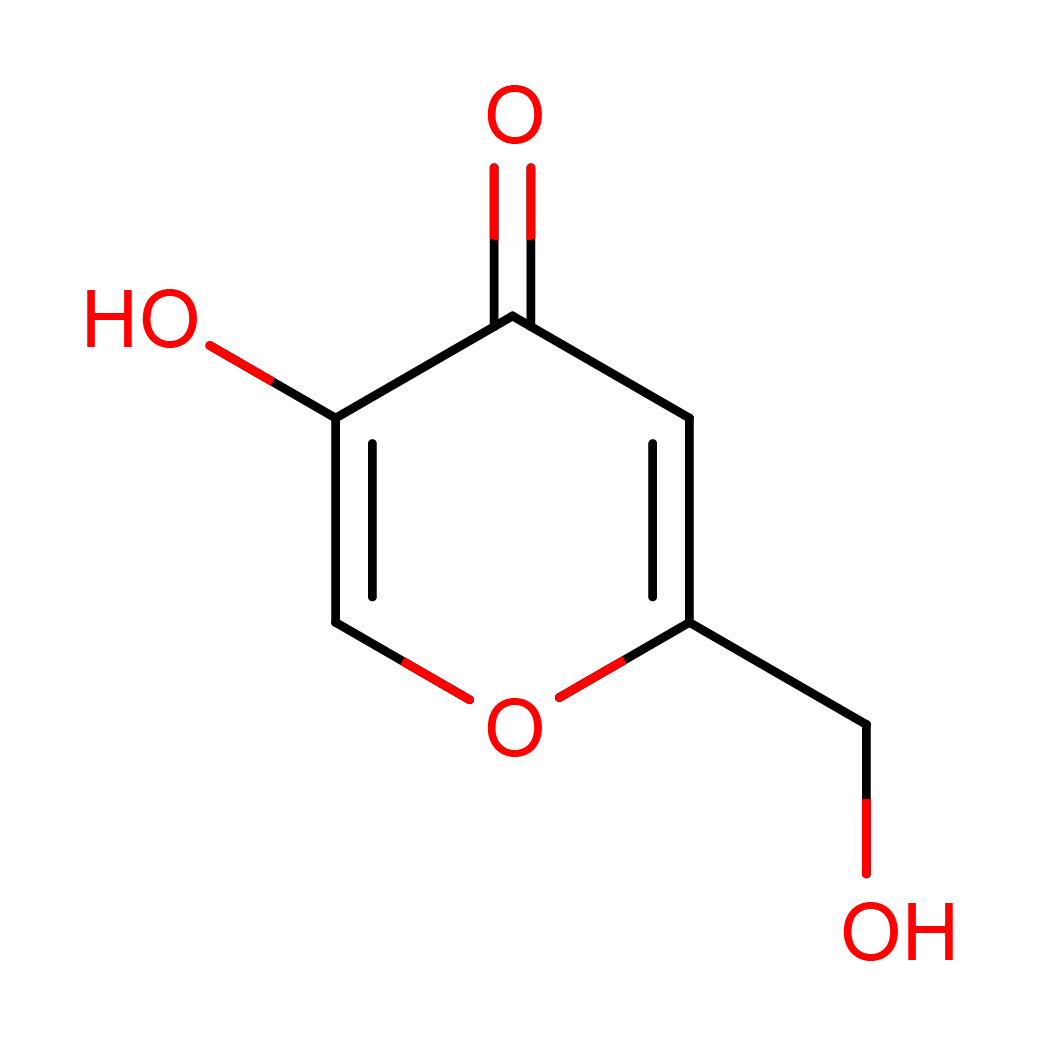
Canonical SMILES: C1=C(OC=C(C1=O)O)CO
Structural Properties:
Molecular Formula: C6H6O4
Molecular Weight: 142.110
Pharmacophore Features:
Number of bond donors: 2
Number of bond acceptors: 4
Number of atoms different from hydrogen: 10
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Fujimoto, N., Watanabe, H., Nakatani, T., Roy, G. and Ito, A., 1998. Induction of thyroid tumours in (C57BL/6NA? C3H/N) F1 mice by oral administration of kojic acid. Food and chemical toxicology, 36(8), pp.697-703. DOI: https://doi.org/10.1016/S0278-6915(98)00030-1. URL: https://www.sciencedirect.com/science/article/pii/S0278691598000301.
Higa, Y., Ohkubo, A., Kitajima, S., Moriyasu, M. and Kariya, K., 2002. Effects of kojic acid on thyroidal functions in rats by single-dose administration and in cultured rat thyroid cells (FRTL-5 cells). The Journal of toxicological sciences, 27(5), pp.423-431. DOI: https://doi.org/10.2131/jts.27.423. URL: https://www.jstage.jst.go.jp/article/jts/27/5/27_5_423/_article/-char/ja/.
Ota, Y., Imai, T., Onose, J.I., Takami, S., Cho, Y.M., Hirose, M. and Nishikawa, A., 2009. A 55-week chronic toxicity study of dietary administered kojic acid (KA) in male F344 rats. The Journal of toxicological sciences, 34(3), pp.305-313. DOI: https://doi.org/10.2131/jts.34.305. URL: https://www.jstage.jst.go.jp/article/jts/34/3/34_3_305/_article/-char/ja/.
External Links


2D-structure
3D-structure