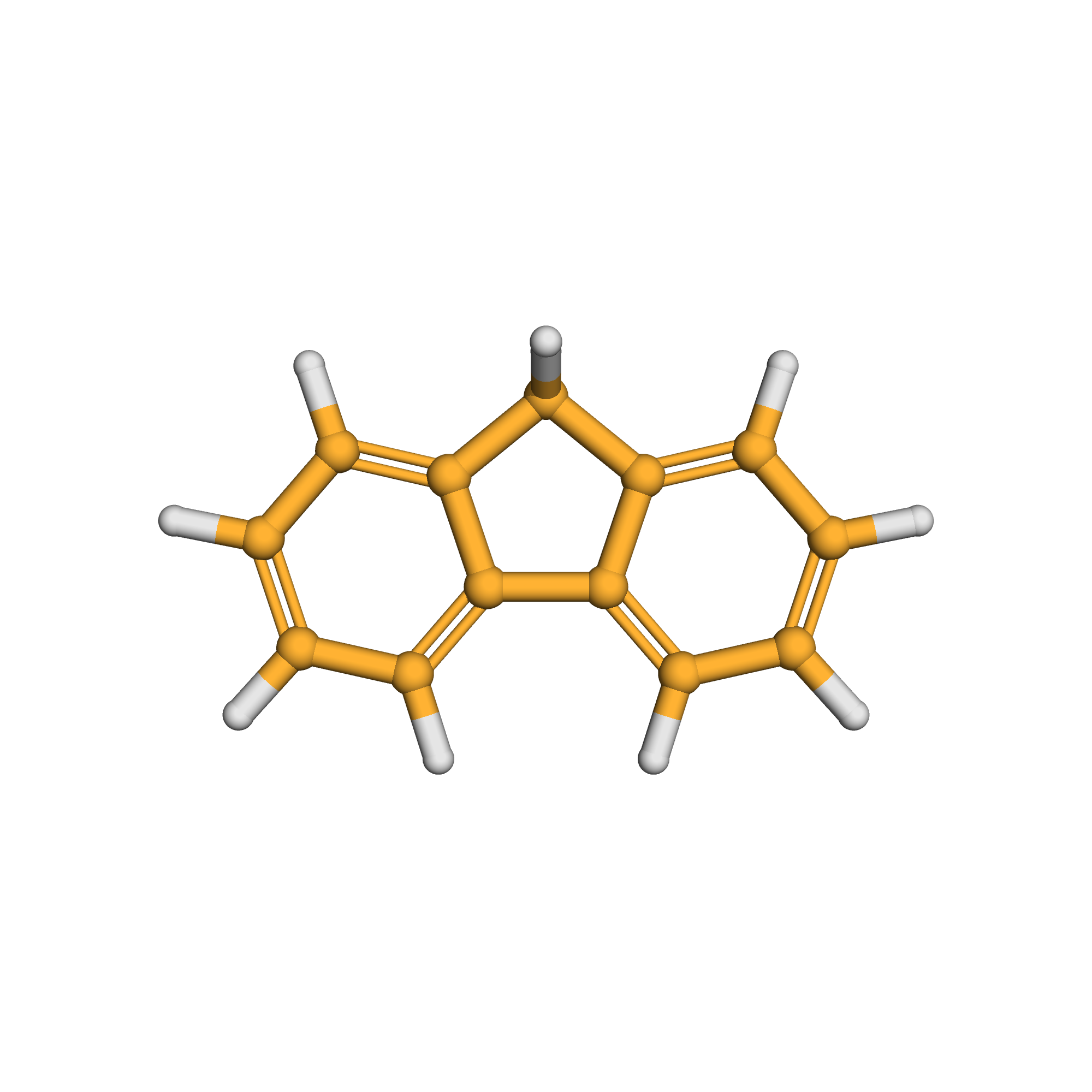
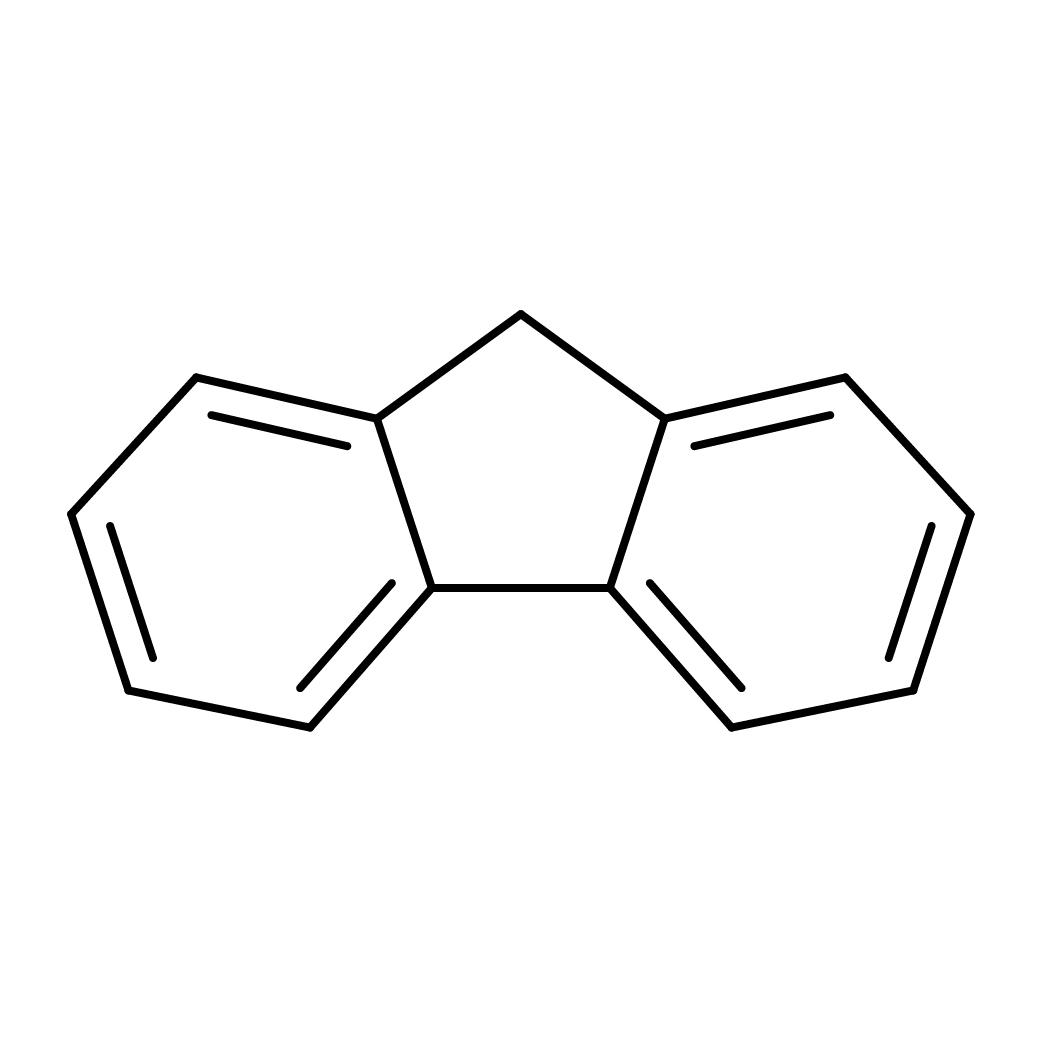
fluorene
Synonyms: "9H-fluorene", "fluoren", "flourene".
Source: Fluorene is used in the production of pharmaceuticals, polymer stabilizers, and dyes.
Identifiers:
IUPAC Name: 9H-fluorene
CAS Number: 86-73-7
PubChem ID: 6853
InChiKey: NIHNNTQXNPWCJQ-UHFFFAOYSA-N
Canonical SMILES: C1C2=CC=CC=C2C3=CC=CC=C31
Structural Properties:
Molecular Formula: C13H10
Molecular Weight: 166.223
Pharmacophore Features:
Number of bond donors: 0
Number of bond acceptors: 0
Number of atoms different from hydrogen: 13
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Incardona JP, Collier TK, Scholz NL. 2004. Defects in cardiac function precede morphological abnormalities in fish embryos exposed to polycyclic aromatic hydrocarbons. Toxicol Appl Pharmacol 196(2):191-205. DOI: 10.1016/j.taap.2003.11.026. URL: https://www.sciencedirect.com/science/article/pii/S0041008X04000110?via%3DihubVondracek J, Kozubak A, Machala M. 2002. Modulation of estrogen receptor-dependent reporter construct activation and G0/G1-S-phase transition by polycyclic aromatic hydrocarbons in human breast carcinoma MCF-7 cells. Toxicol Sci 70(2):193-201. DOI: 10.1093/toxsci/70.2.193. URL: https://academic.oup.com/toxsci/article/70/2/193/1621654.
External Links



2D-structure
3D-structure