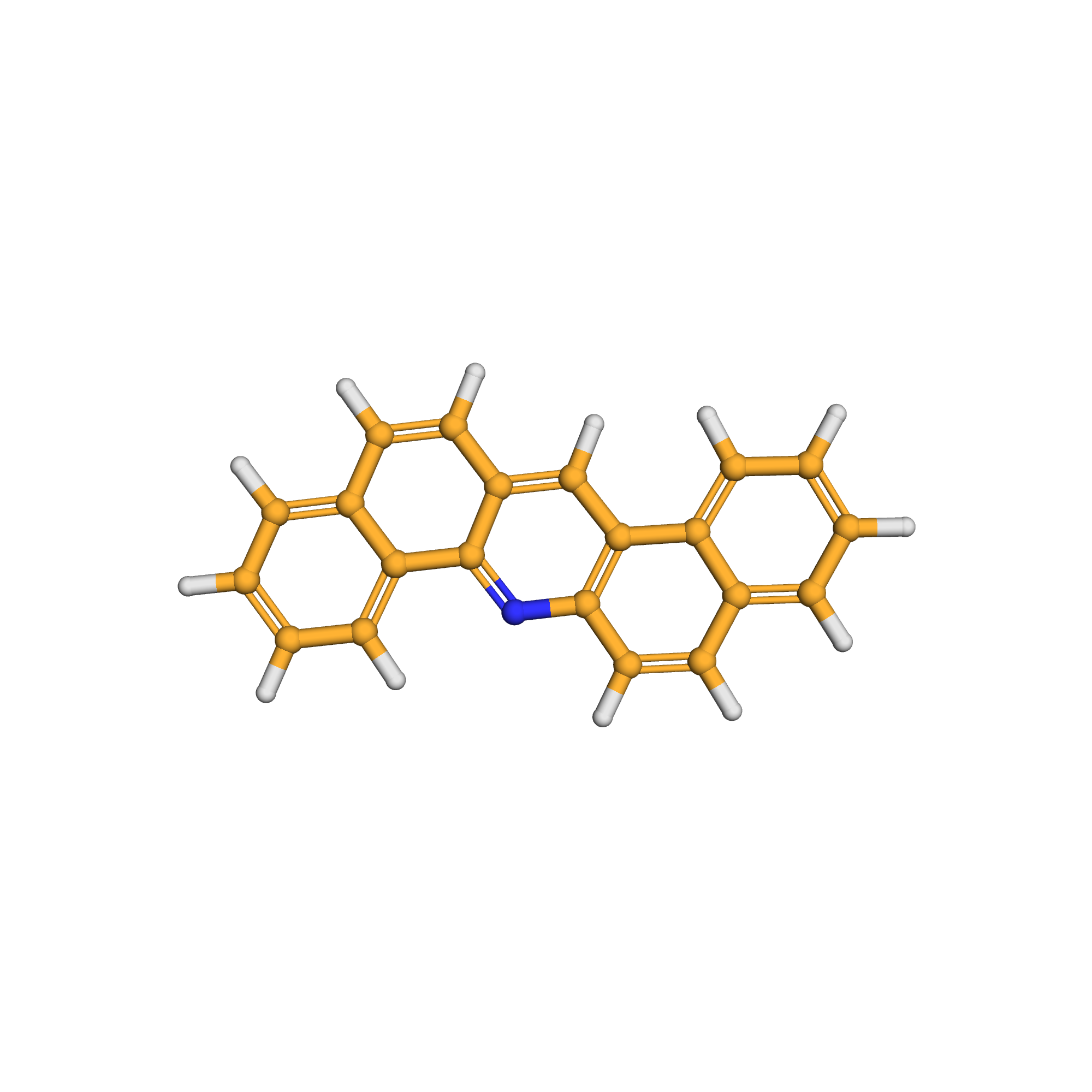
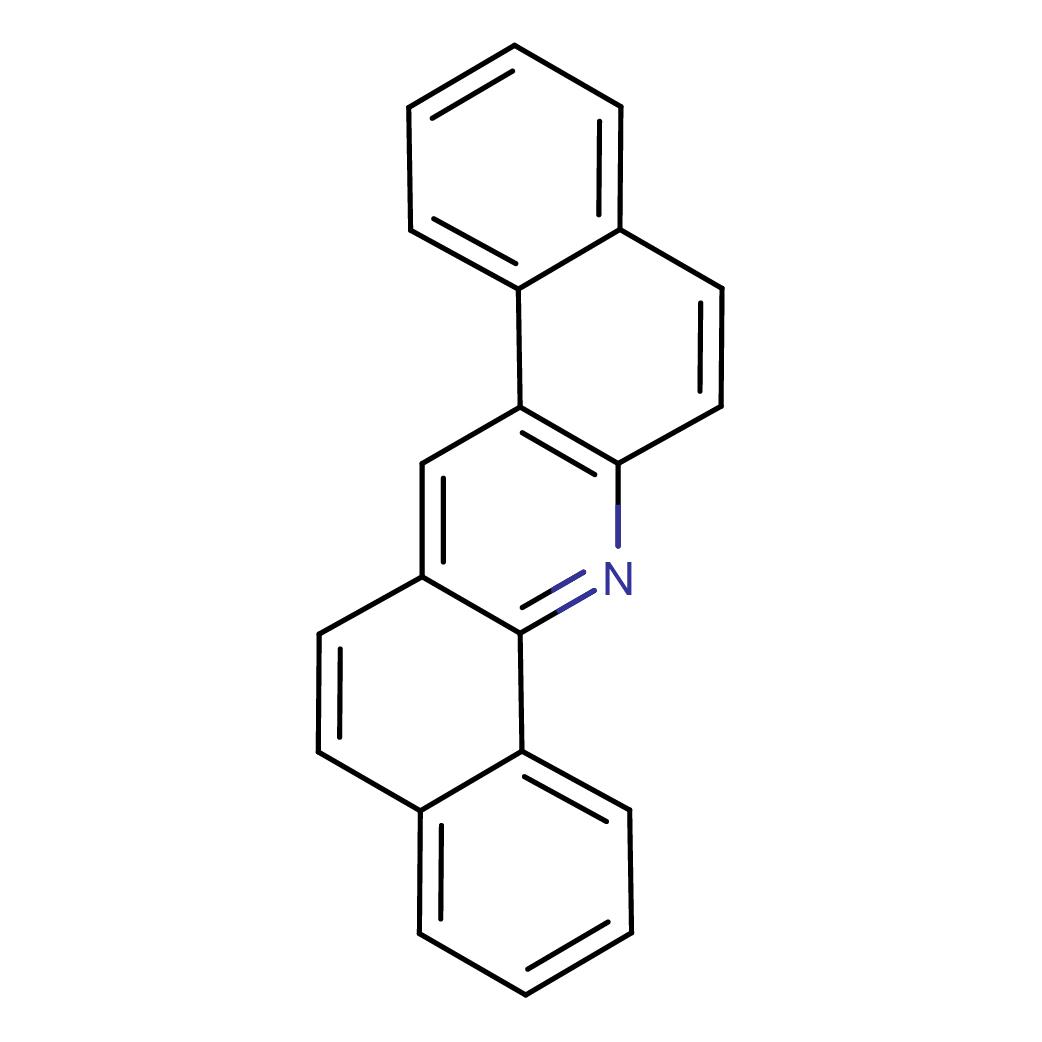
dibenz[a,h]acridine
Synonyms: "dibenz[a,h]acridine", "dibenz(A,H)acridine", "dibenzo[a,H]acridine", "dibenz(a,d)acridine", "1,2,5,6-dibenzacridine", "7-azadibenz(a,h)anthracene", "DB(a,h)AC", "1,2,5,6-dibenzoacridine", "1,2,5,6-dinaphthacridine", "7-azadibenz[A,H]antracene", "dibenz[a,d]acridine", "7-azadibenz[a,h]anthracene".
Source: Dibenz[a,h]acridine is formed during the incomplete burning of organic matter.
Identifiers:
IUPAC Name: 2-azapentacyclo[12.8.0.03,12.04,9.015,20]docosa-1(14),2,4,6,8,10,12,15,17,19,21-undecaene
CAS Number: 226-36-8
PubChem ID: 9183
InChiKey: JNCSIWAONQTVCF-UHFFFAOYSA-N
Canonical SMILES: C1=CC=C2C(=C1)C=CC3=C2C=C4C=CC5=CC=CC=C5C4=N3
Structural Properties:
Molecular Formula: C21H13N
Molecular Weight: 279.342
Pharmacophore Features:
Number of bond donors: 0
Number of bond acceptors: 1
Number of atoms different from hydrogen: 22
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Benisek M, Blaha L, Hilscherova K. 2008. Interference of PAHs and their N-heterocyclic analogs with signaling of retinoids in vitro. Toxicol in Vitro 22(8):1909-1917. DOI: 10.1016/j.tiv.2008.09.009. URL: http://www.sciencedirect.com/science/article/pii/S0887233308002373?via%3Dihub.
External Links


2D-structure
3D-structure