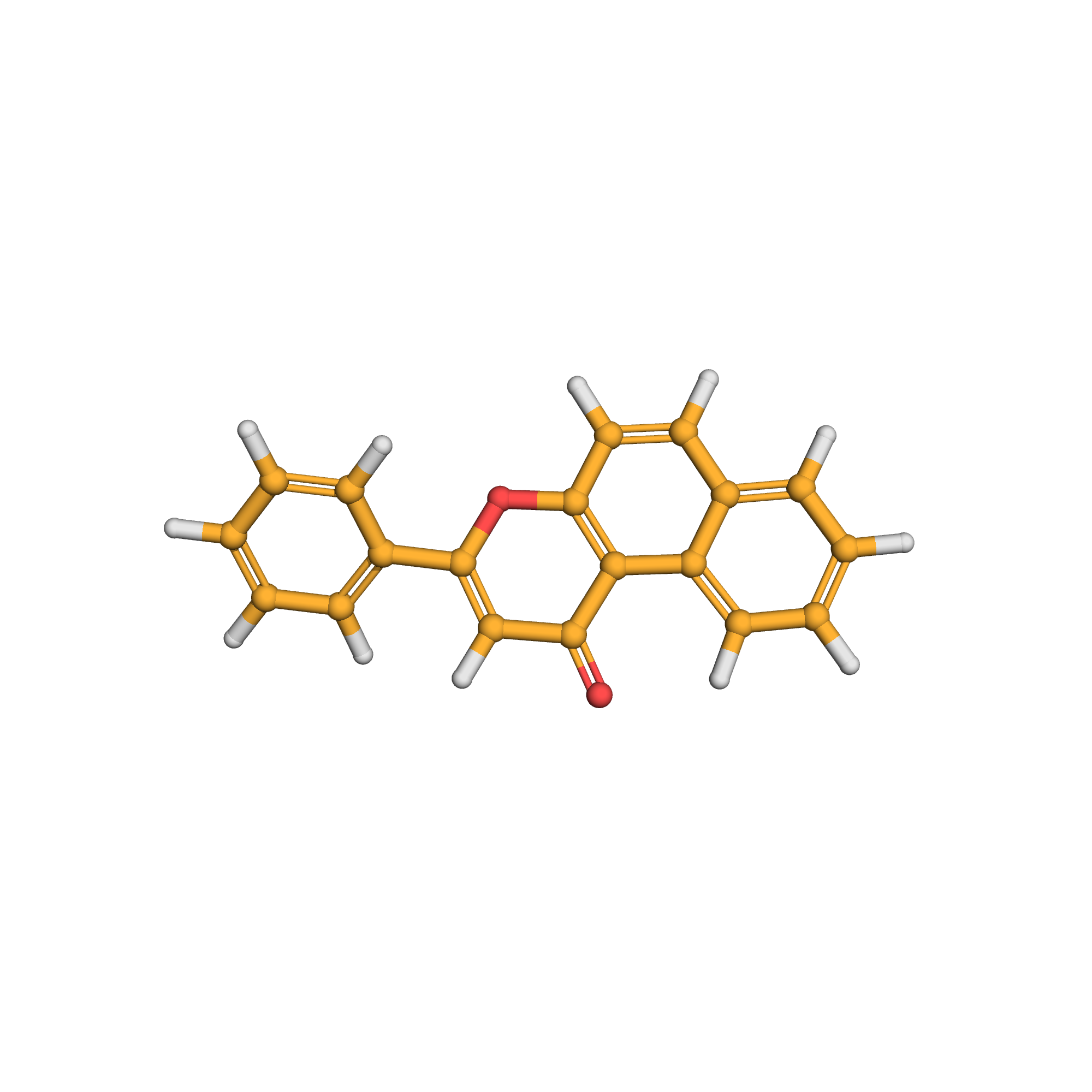
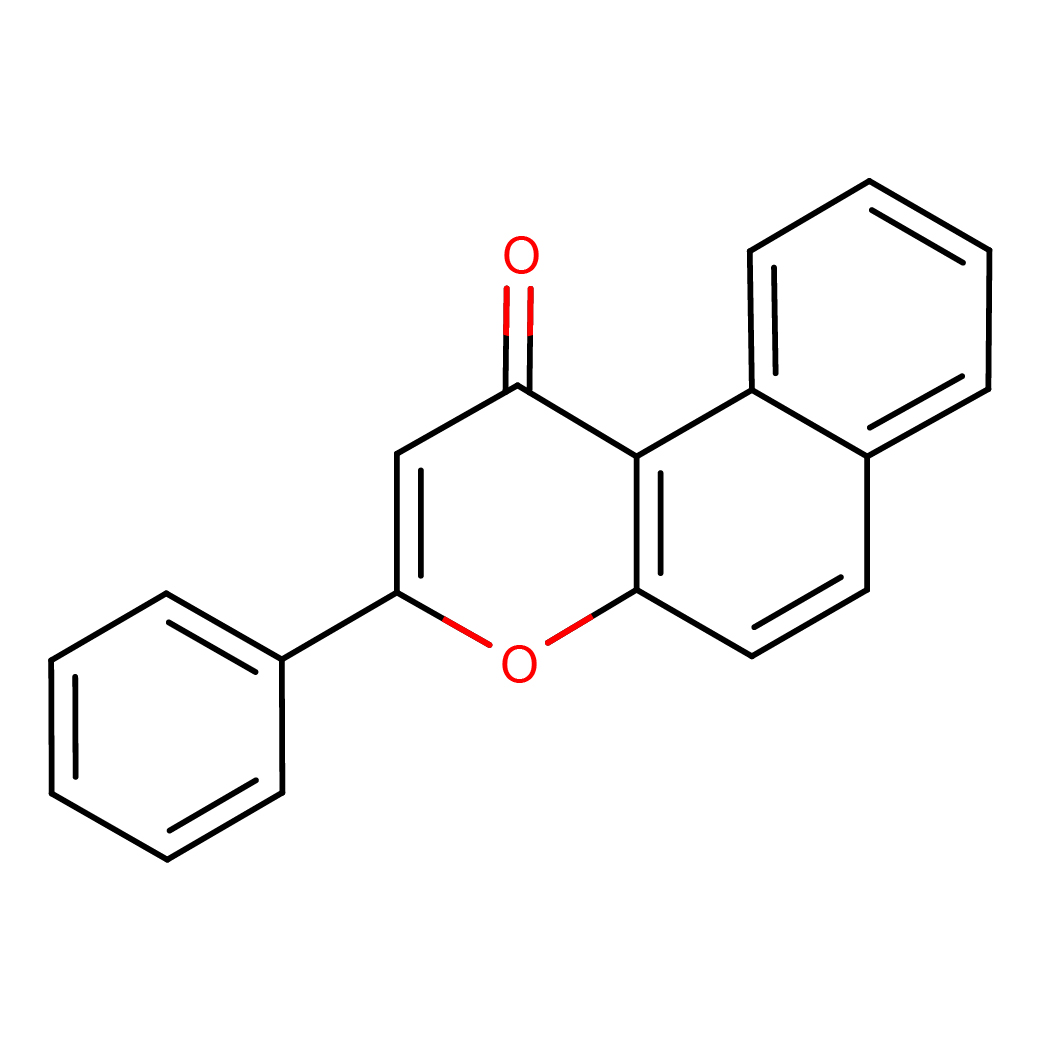
beta-naphthoflavone
Synonyms: "5,6-benzoflavone", "beta-NF", "3-Phenyl-1H-naphtho(2,1-b)pyran-1-one", "b-naphthoflavone", "3-phenyl-1H-benzo[f]chromen-1-one", "3-phenylbenzo[f]chromen-1-one", "3-phenyl-1H-naphtho[2,1-b]pyran-1-one", "3-phenyl-benzo[f]chromen-1-one".
Source: Beta-naphthoflavone is a reference chemical known to be an effective in- ducer of CYP1A. It differs from naturally occurring flavones in having an additional condensed ring and also in lacking either hydroxy or methoxy substit uents.
Identifiers:
IUPAC Name: 3-phenylbenzo[f]chromen-1-one
CAS Number: 6051-87-2
PubChem ID: 2361
InChiKey: OUGIDAPQYNCXRA-UHFFFAOYSA-N
Canonical SMILES: C1=CC=C(C=C1)C2=CC(=O)C3=C(O2)C=CC4=CC=CC=C43
Structural Properties:
Molecular Formula: C19H12O2
Molecular Weight: 272.303
Pharmacophore Features:
Number of bond donors: 0
Number of bond acceptors: 2
Number of atoms different from hydrogen: 21
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Evanson M, Van Der Kraak GJ. 2001. Stimulatory effects of selected PAHs on testosterone production in goldfish and rainbow trout and possible mechanisms of action. Comp Biochem Physiol C Toxicol Pharmacol 130(2):249-258. DOI: 10.1016/S1532-0456(01)00246-0. URL: https://www.sciencedirect.com/science/article/pii/S1532045601002460.
External Links

2D-structure
3D-structure