4-tert-butylcatechol
Synonyms: "p-tert-butylcatechol", "4-(tert-butyl)benzene-1,2-diol", "4-tert-butylpyrocatechol", "4-tert-butylbenzene-1,2-diol", "p-tert-butyl catechol", "4-t-butylpyrocatechol", "p-tert-butylpyrocatechol", "p-t-butylpyrocatechol", "4-tert-butylcatechin", "t-butyl catechol", "Synox TBC", "4-tert-Butyl-1,2-benzenediol", "4-TBC", "1,2-dihydroxy-4-tert-butylbenzene", "4-tert-butyl-1,2-dihydroxybenzene", "4-tert-butylpyrokatechin".
Source: 4-tert-Butylcatechol is usually added as a stabilizer and an inhibitor of polymerization to butadiene, styrene and other reactive monomer streams.
Identifiers:
IUPAC Name: 4-tert-butylbenzene-1,2-diol
CAS Number: 98-29-3
PubChem ID: 7381
InChiKey: XESZUVZBAMCAEJ-UHFFFAOYSA-N
Canonical SMILES: CC(C)(C)C1=CC(=C(C=C1)O)O
Structural Properties:
Molecular Formula: C10H14O2
Molecular Weight: 166.220
Pharmacophore Features:
Number of bond donors: 2
Number of bond acceptors: 2
Number of atoms different from hydrogen: 12
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Akahori Y, Nakai M, Yamasaki K, Takatsuki M, Shimohigashi Y, Ohtaki M. 2008. Relationship between the results of in vitro receptor binding assay to human estrogen receptor alpha and in vivo uterotrophic assay: Comparative study with 65 selected chemicals. Toxicol in Vitro 22(1):225-231. DOI: 10.1016/j.tiv.2007.08.004. URL: http://www.sciencedirect.com/science/article/pii/S0887233307002251?via%3Dihub.
External Links


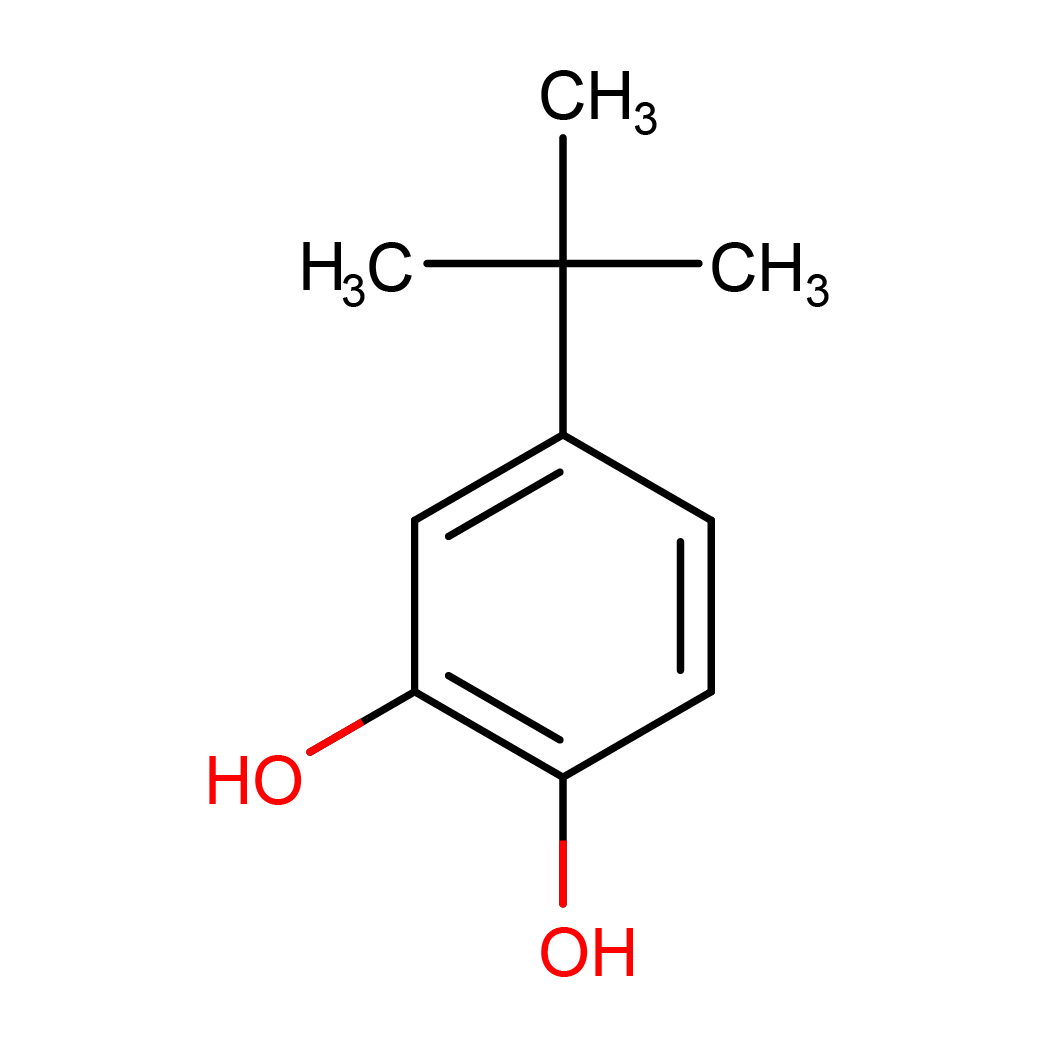
2D-structure
3D-structure