4,4'-(1,1-dimethyl-3-methylene-1,3-propanediyl)bis-phenol
Synonyms: "4-methyl-2,4-bis(4-hydroxyphenyl)pent-1-ene", "4,4'-(4-methylpent-1-ene-2,4-diyl)diphenol", "4-methyl-2,4-bis(p-hydroxyphenyl)pent-1-ene", "2,4-bis(4-hydroxyphenyl)-4-methyl-1-pentene", "4-methyl-2,4-bis-(p-hydroxyphenyl)pent-1-ene".
Source: 4-Methyl-2,4-bis(p-hydroxyphenyl)pent-1-ene (MBP) is a metabolite of bisphenol A.
Identifiers:
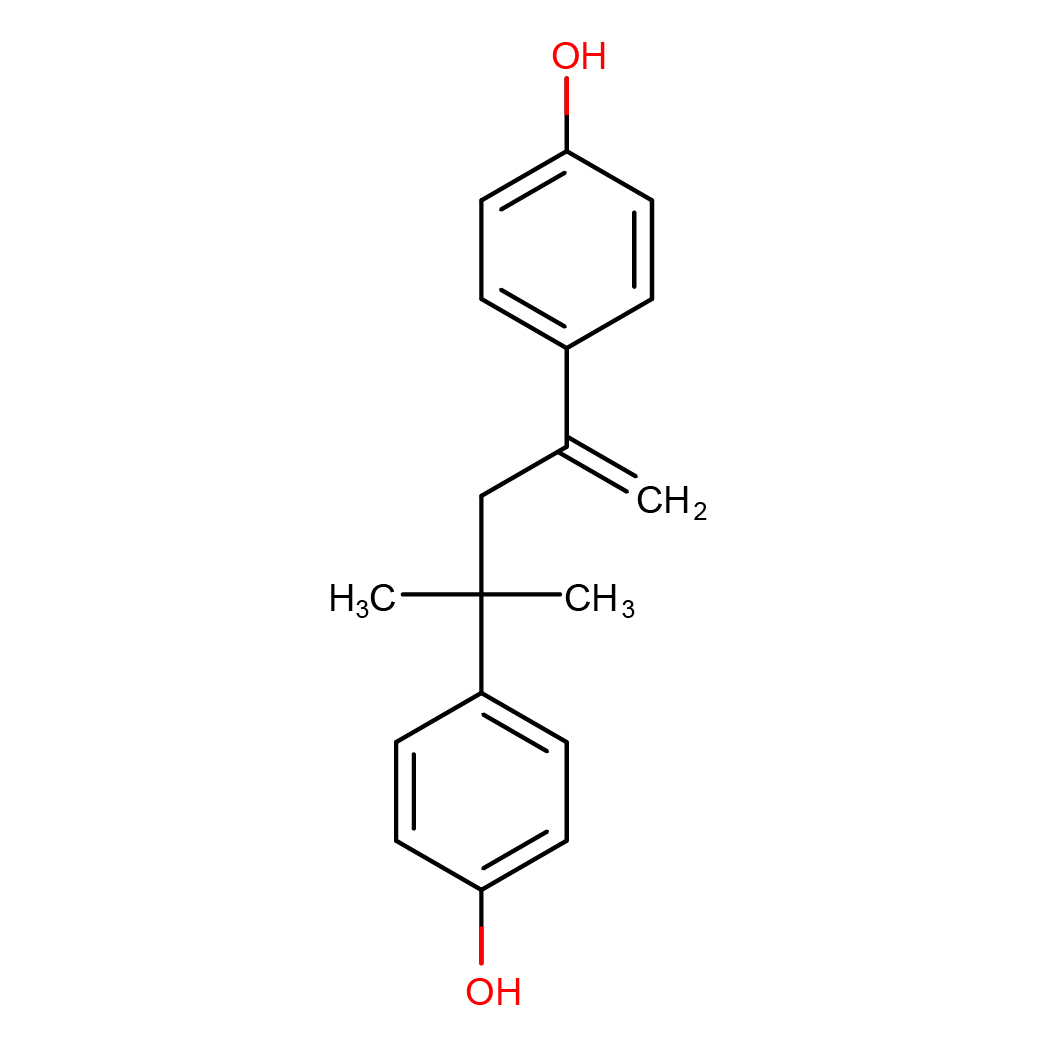
IUPAC Name: 4-[4-(4-hydroxyphenyl)-4-methylpent-1-en-2-yl]phenol
CAS Number: 13464-24-9
PubChem ID: 83494
InChiKey: MZLYLGGRVAFGBY-UHFFFAOYSA-N
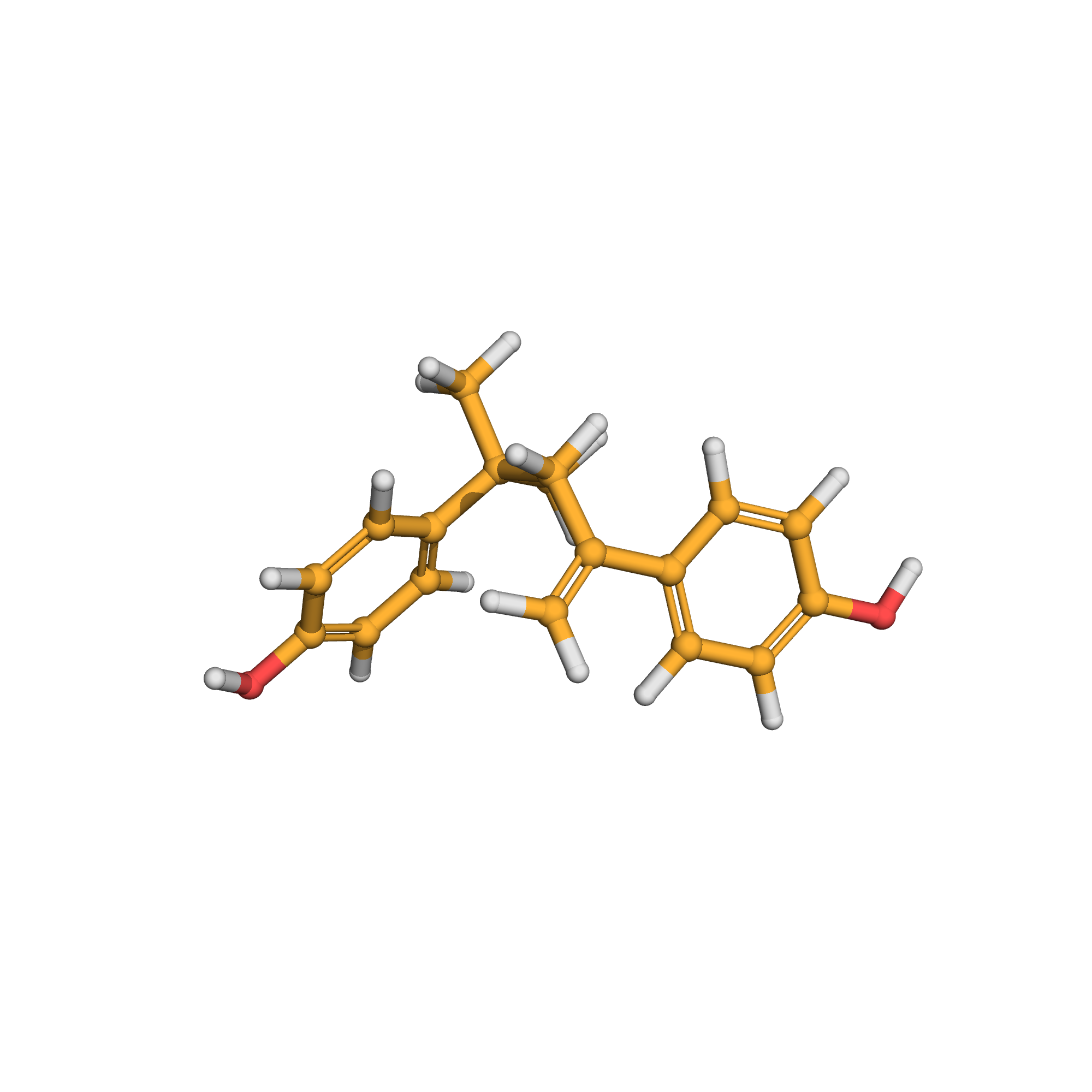
Canonical SMILES: CC(C)(CC(=C)C1=CC=C(C=C1)O)C2=CC=C(C=C2)O
Structural Properties:
Molecular Formula: C18H20O2
Molecular Weight: 268.356
Pharmacophore Features:
Number of bond donors: 2
Number of bond acceptors: 2
Number of atoms different from hydrogen: 20
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Ishibashi H, Watanabe N, Matsumura N, Hirano M, Nagao Y, Shiratsuchi H, Kohra S, Yoshihara S, Arizono K. 2005. Toxicity to early life stages and an estrogenic effect of a bisphenol A metabolite, 4-methyl-2,4-bis(4-hydroxyphenyl)pent-1-ene on the medaka (Oryzias latipes). Life Sci 77(21):2643-2655. DOI: 10.1016/j.lfs.2005.03.025. URL: https://www.sciencedirect.com/science/article/pii/S002432050500528X?via%3Dihub.
Nagae M, Shiroyama K, Inoue M, Hara A, Takao Y, Kohra S, Ishibashi Y, Tominaga N, Yoshihara S, Arizono K. 2005. Estrogenic potency of a bisphenol A metabolite on vitellogenin synthesis in medaka, Oryzias latipes. Journal of Health Science 51(1):93-95. DOI: 10.1248/jhs.51.93. URL: https://www.jstage.jst.go.jp/article/jhs/51/1/51_1_93/_article.
Okuda K, Takiguchi M, Yoshihara S. 2010. In vivo estrogenic potential of 4-methyl-2,4-bis(4-hydroxyphenyl)pent-1-ene, an active metabolite of bisphenol A, in uterus of ovariectomized rat. Toxicol Lett 197(1):7-11. DOI: 10.1016/j.toxlet.2010.04.017. URL: https://www.sciencedirect.com/science/article/pii/S0378427410014694?via%3Dihub.
Yamaguchi A, Ishibashi H, Kohra S, Arizono K, Tominaga N. 2005. Short-term effects of endocrine-disrupting chemicals on the expression of estrogen-responsive genes in male medaka (Oryzias latipes). Aquat Toxicol 72(3):239-249. DOI: 10.1016/j.aquatox.2004.12.011. URL: https://www.sciencedirect.com/science/article/pii/S0166445X05000123.
Yoshihara S, Mizutare T, Makishima M, Suzuki N, Fujimoto N, Igarashi K, Ohta S. 2004. Potent estrogenic metabolites of bisphenol A and bisphenol B formed by rat liver S9 fraction: their structures and estrogenic potency. Toxicol Sci 78(1):50-59. DOI: 10.1093/toxsci/kfh047. URL: https://academic.oup.com/toxsci/article/78/1/50/1625641.
External Links

2D-structure
3D-structure