4,4'-dihydroxybenzophenone
Synonyms: "4,4'-dihydroxybenzophenone", "bis(4-hydroxyphenyl)methanone", "bis(4-hydroxyphenyl) ketone", "4,4'-dihydoxy-benzophenone", "Bis(p-hydroxy)benzophenone", "4,4'-dihydroxydiphenyl ketone", "p,p'-dihydroxybenzophenone", "4,4'-dihydoxybenzophenone", "4-(4-hydroxybenzoyl)phenol", "4,4'-dihydroxy benzophenone".
Source: 4,4'-dihydroxybenzophenone is used as an intermediate for medicines, dyes, pesticides, ultraviolet absorbers, etc.
Identifiers:
IUPAC Name: bis(4-hydroxyphenyl)methanone
CAS Number: 611-99-4
PubChem ID: 69150
InChiKey: RXNYJUSEXLAVNQ-UHFFFAOYSA-N
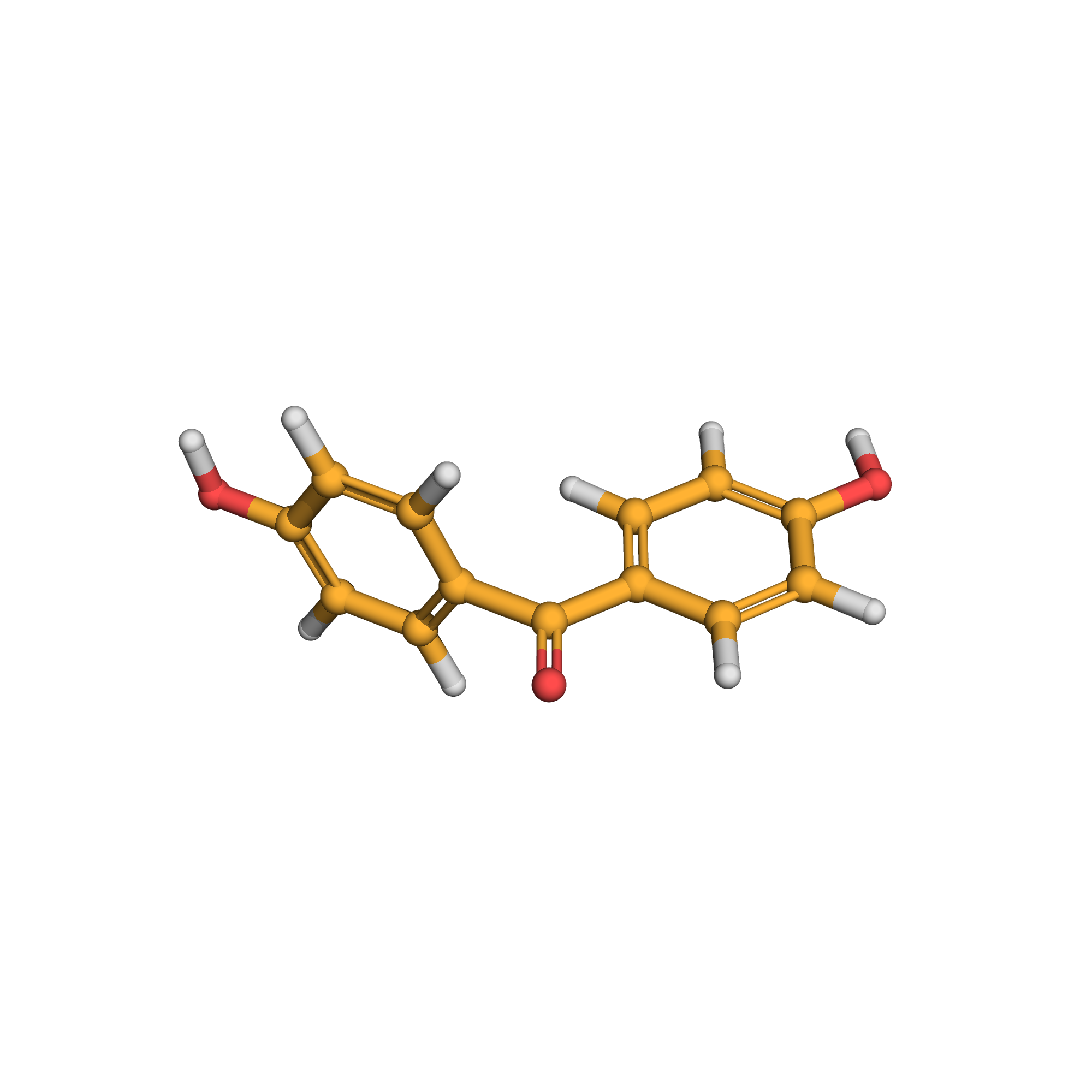
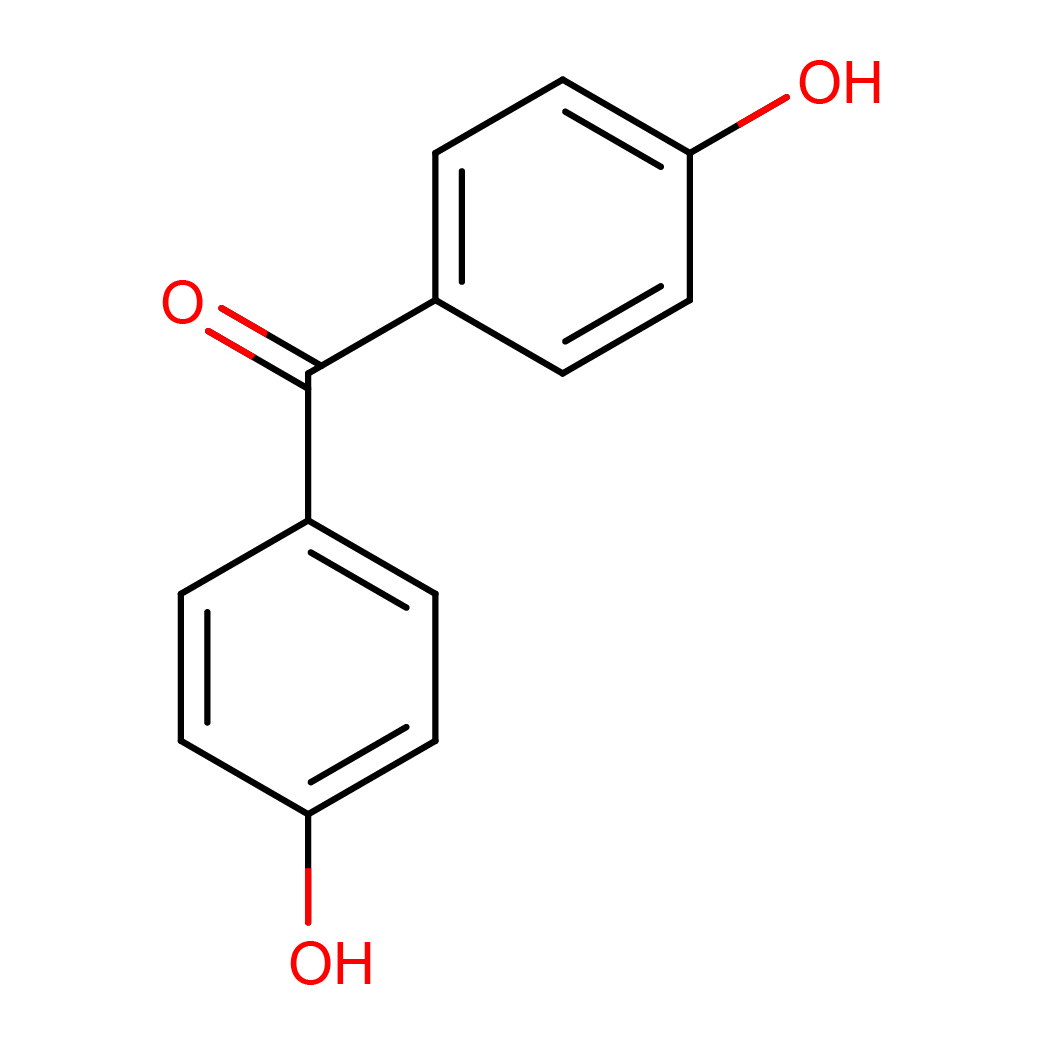
Canonical SMILES: C1=CC(=CC=C1C(=O)C2=CC=C(C=C2)O)O
Structural Properties:
Molecular Formula: C13H10O3
Molecular Weight: 214.220
Pharmacophore Features:
Number of bond donors: 2
Number of bond acceptors: 3
Number of atoms different from hydrogen: 16
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Heneweer M, Muusse M, van den Berg M, Sanderson JT. 2005. Additive estrogenic effects of mixtures of frequently used UV filters on pS2-gene transcription in MCF-7 cells. Toxicol Appl Pharmacol 208(2):170-177. DOI: 10.1016/j.taap.2005.02.006. URL: https://www.sciencedirect.com/science/article/pii/S0041008X05000669.
Kunz PY, Fent K. 2006. Multiple hormonal activities of UV filters and comparison of in vivo and in vitro estrogenic activity of ethyl-4-aminobenzoate in fish. Aquat Toxicol 79(4):305-324. DOI: 10.1016/j.aquatox.2006.06.016. URL: https://www.sciencedirect.com/science/article/pii/S0166445X06002700.
Kunz PY, Galicia HF, Fent K. 2006. Comparison of in vitro and in vivo estrogenic activity of UV filters in fish. Toxicol Sci 90(2):349-361 DOI: 10.1093/toxsci/kfj082. URL: https://academic.oup.com/toxsci/article/90/2/349/1658390.
Schultz TW, Seward JR, Sinks GD. 2000. Estrogenicity of benzophenones evaluated with a recombinant yeast assay: comparison of experimental and rules-based predicted activity. Environ Toxicol Chem 19(2):301-304. DOI: 10.1002/etc.5620190208. URL: http://onlinelibrary.wiley.com/doi/10.1002/etc.5620190208/abstract.
Suzuki T, Kitamura S, Khota R, Sugihara K, Fujimoto N, Ohta S. 2005. Estrogenic and antiandrogenic activities of 17 benzophenone derivatives used as UV stabilizers and sunscreens. Toxicol Appl Pharmacol 203(1):9-17. DOI: 10.1016/j.taap.2004.07.005. URL: https://www.sciencedirect.com/science/article/pii/S0041008X04003539.
Yamasaki K, Takeyoshi M, Sawaki M, Imatanaka N, Shinoda K, Takatsuki M. 2003. Immature rat uterotrophic assay of 18 chemicals and Hershberger assay of 30 chemicals. Toxicology 183(1-3):93-115. DOI: 10.1016/S0300-483X(02)00445-6. URL: https://www.sciencedirect.com/science/article/pii/S0300483X02004456?via%3Dihub.
Yamasaki K, Takeyoshi M, Yakabe Y, Sawaki M, Takatsuki M. 2003. Comparison of the reporter gene assay for ERalpha antagonists with the immature rat uterotrophic assay of 10 chemicals. Toxicol Lett 142(1-2):119-131. DOI: 10.1016/S0378-4274(03)00019-5. URL: https://www.sciencedirect.com/science/article/pii/S0378427403000195?via%3Dihub.
External Links

2D-structure
3D-structure