2,6-di-tert-butyl-4-methylphenol
Synonyms: "2,6-Di-tert-butyl-4-methylphenol", "butylated hydroxytoluene", "butylhydroxytoluene", "2,6-di-tert-butyl-p-cresol", "2,6-di-t-butyl-4-methylphenol", "Ionol", "DBPC", "BHT", "Stavox", "Dibunol", "Ionol CP", "Impruvol", "Topanol", "Ionole", "Deenax", "Vianol", "Dalpac", "Antioxidant KB", "3,5-Di-tert-butyl-4-hydroxytoluene", "Topanol O", "Antioxidant 4K", "Sumilizer BHT", "Vanlube PC", "Topanol OC", "Tenamene 3", "Antioxidant DBPC", "Sustane BHT", "Vanlube PCX", "Antioxidant 30", "Antioxidant 29", "Nonox TBC", "Tenox BHT", "Chemanox 11", "Ionol 1", "Agidol", "Catalin CAO-3", "Ionol (antioxidant)", "Advastab 4".
Source: 2,6-di-tert-butyl-4-methylphenol is a widely used antioxidant in the production of polyethylene-
Identifiers:
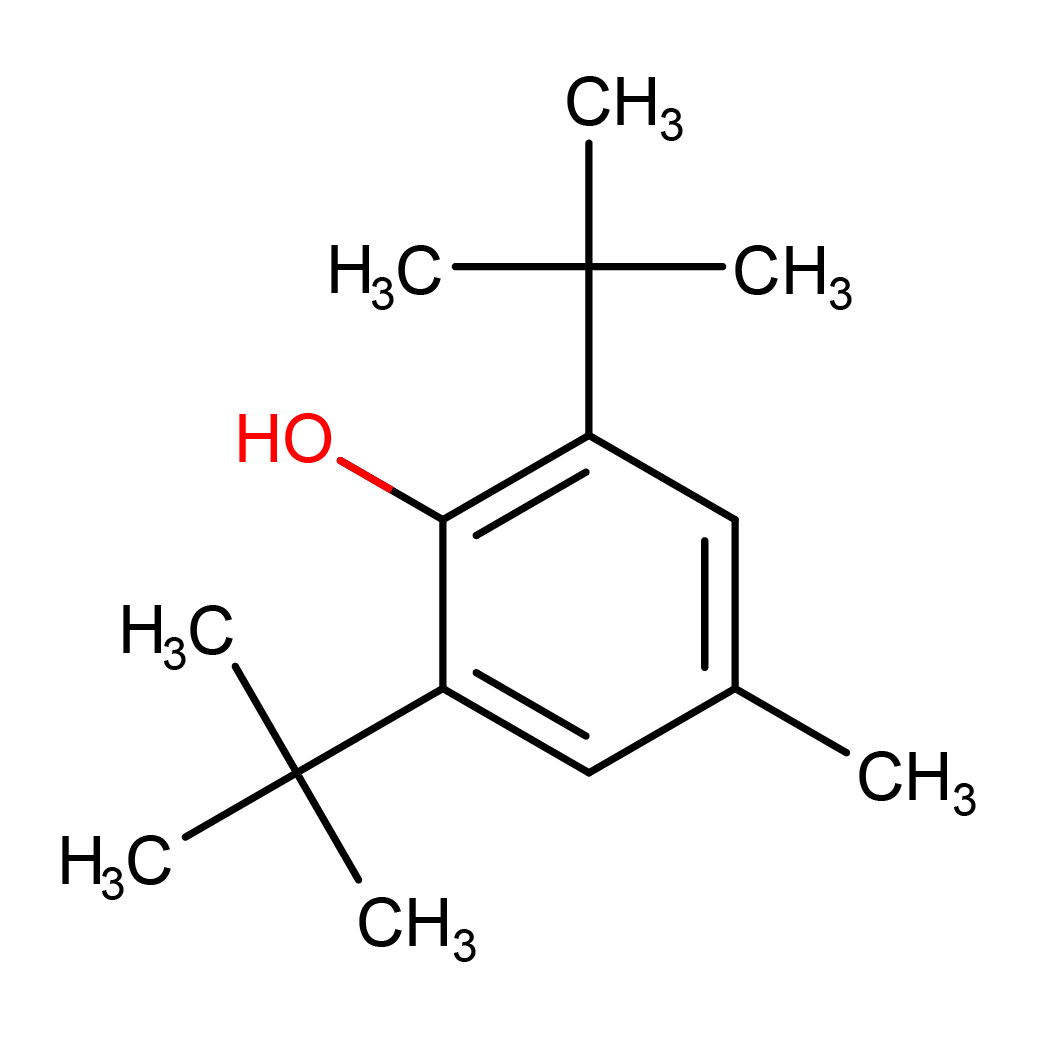
IUPAC Name: 2,6-ditert-butyl-4-methylphenol
CAS Number: 128-37-0
PubChem ID: 31404
InChiKey: NLZUEZXRPGMBCV-UHFFFAOYSA-N
Canonical SMILES: CC1=CC(=C(C(=C1)C(C)(C)C)O)C(C)(C)C
Structural Properties:
Molecular Formula: C15H24O
Molecular Weight: 220.356
Pharmacophore Features:
Number of bond donors: 1
Number of bond acceptors: 1
Number of atoms different from hydrogen: 16
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Hughes PJ, McLellan H, Lowes DA, Khan SZ, Bilmen JG, Tovey SC, Godfrey RE, Michell RH, Kirk CJ, Michelangeli F. 2000. Estrogenic alkylphenols induce cell death by inhibiting testis endoplasmic reticulum Ca2+ pumps. Biochem Biophys Res Commun 277(3):568-574. DOI: 10.1006/bbrc.2000.3710. URL: http://www.sciencedirect.com/science/article/pii/S0006291X00937100?via%3Dihub.
External Links

2D-structure
3D-structure