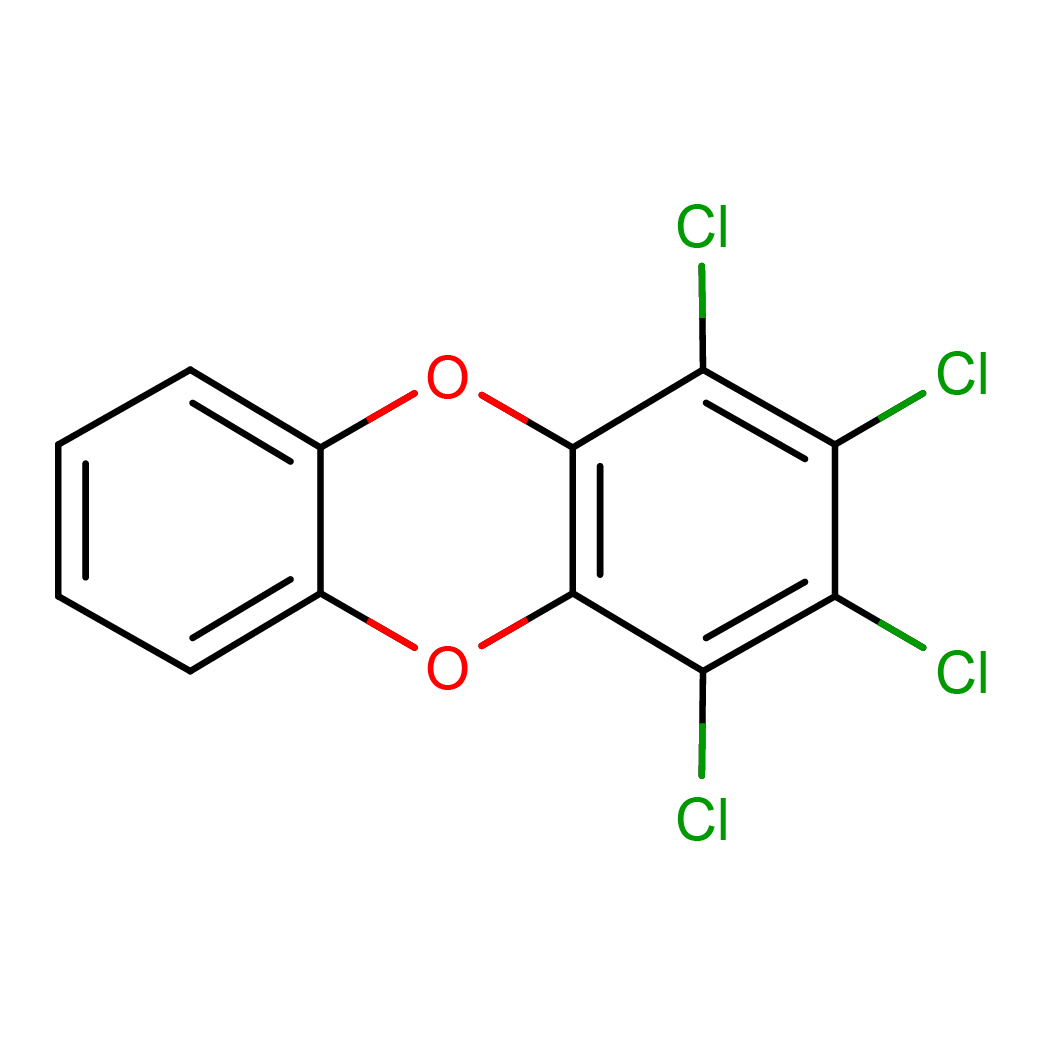
1,2,3,4-tetrachlorodibenzo-p-dioxin
Synonyms: "1,2,3,4-tetrachlorodibenzo-p-dioxin", "1,2,3,4-tetrachlorodibenzodioxin", "1,2,3,4-tetrachlorodibenzo-para-dioxin", "1,2,3,4-TCDD", "1,2,3,4-tetrachlorooxanthrene", "1,2,3,4-tetrachlorodibenzodioxine", "1,2,3,4-tetrachlorodibenzo[b,e][1,4]dioxin", "1,2,3,4-tetrachloro(13c12)oxanthrene".
Source: 1,2,3,4-tetrachlorodibenzo-p-dioxin is a polychlorinated dibenzodioxin (PCDDs). PCDDs occur as by-products in the manufacture of some organochlorines, in the incineration of chlorine-containing substances such as PVC (polyvinyl chloride), in the chlorine bleaching of paper, and from natural sources such as volcanoes and forest fires.
Identifiers:
IUPAC Name: 1,2,3,4-tetrachlorodibenzo-p-dioxin
CAS Number: 30746-58-8
PubChem ID: 35454
InChiKey: DJHHDLMTUOLVHY-UHFFFAOYSA-N
Canonical SMILES: C1=CC=C2C(=C1)OC3=C(O2)C(=C(C(=C3Cl)Cl)Cl)Cl
Structural Properties:
Molecular Formula: C12H4Cl4O2
Molecular Weight: 321.962
Pharmacophore Features:
Number of bond donors: 0
Number of bond acceptors: 2
Number of atoms different from hydrogen: 18
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Mason G, Farrell K, Keys B, Piskorska-Pliszczynska J, Safe L, Safe S. 1986. Polychlorinated dibenzo-p-dioxins: Quantitative in vitro and in vivo structure-activity relationships. Toxicology 41(1):21-31. DOI: 10.1016/0300-483X(86)90101-0. URL: https://www.sciencedirect.com/science/article/pii/0300483X86901010.
External Links




2D-structure
3D-structure