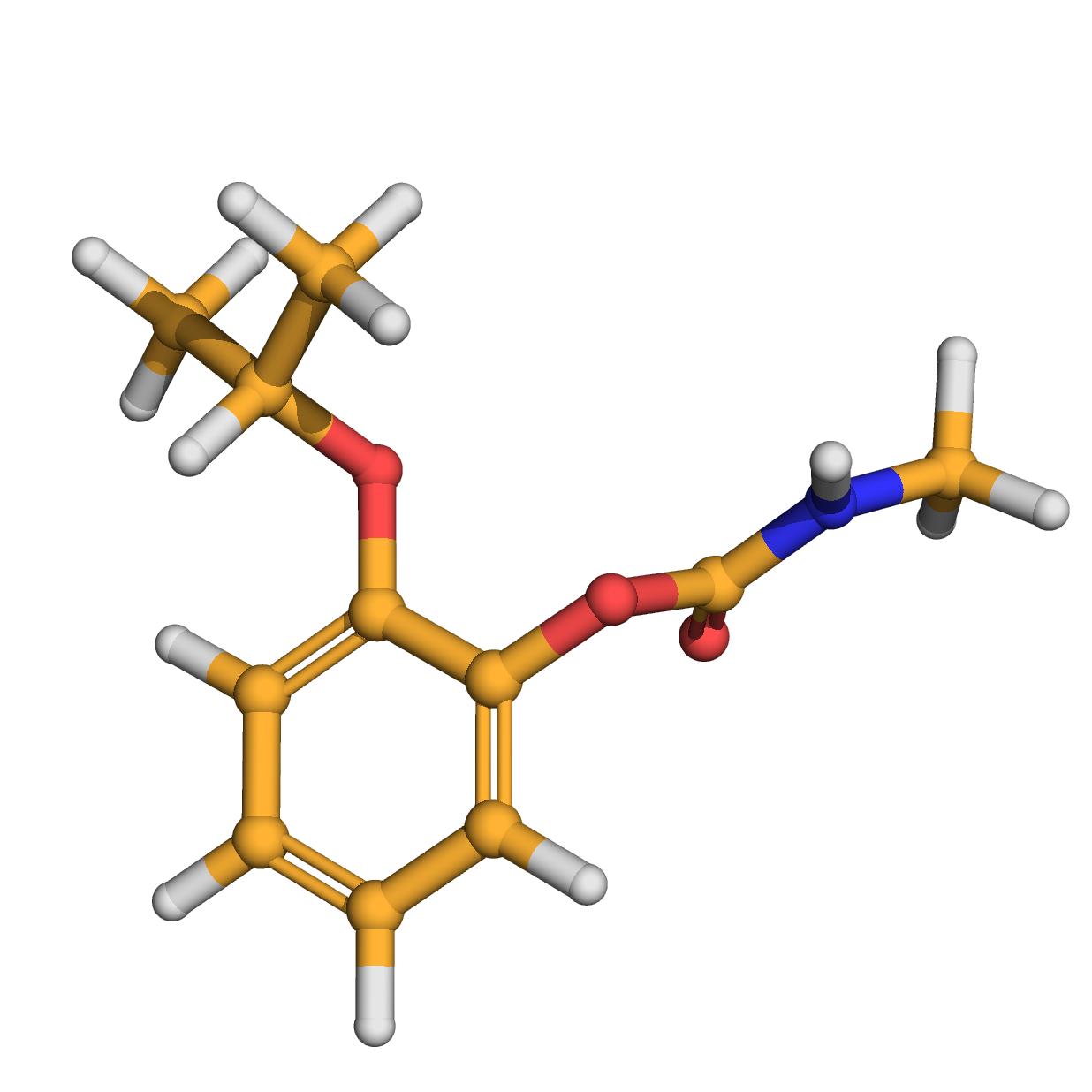
propoxur
Synonyms: "baygon", "aprocarb", "sendran", "propoxure", "blattanex", "blattosep", "mrowkozol", "propotox", "propoxylor"
Source: propoxur is an insecticide used to control cockroaches, flies, mosquitoes, and lawn insects.
Identifiers:
IUPAC Name: (2-propan-2-yloxyphenyl) N-methylcarbamate
CAS Number: 114-26-1
PubChem ID: 4944
InChiKey: ISRUGXGCCGIOQO-UHFFFAOYSA-N
Canonical SMILES: CC(C)OC1=CC=CC=C1OC(=O)NC
Structural Properties:
Molecular Formula: C11H15NO3
Molecular Weight: 209.242
Pharmacophore Features:
Number of bond donors: 1
Number of bond acceptors: 2
Number of atoms different from hydrogen: 15
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Klotz DM, Arnold SF, McLachlan JA. 1997. Inhibition of 17 beta-estradiol and progesterone activity in human breast and endometrial cancer cells by carbamate insecticides. Life Sci 60(17):1467-1475.
Schmuck G, Mihail F. 2004. Effects of the carbamates fenoxycarb, propamocarb and propoxur on energy supply, glucose utilization and SH-groups in neurons. Arch Toxicol 78(6):330-337.
External Links

2D-structure
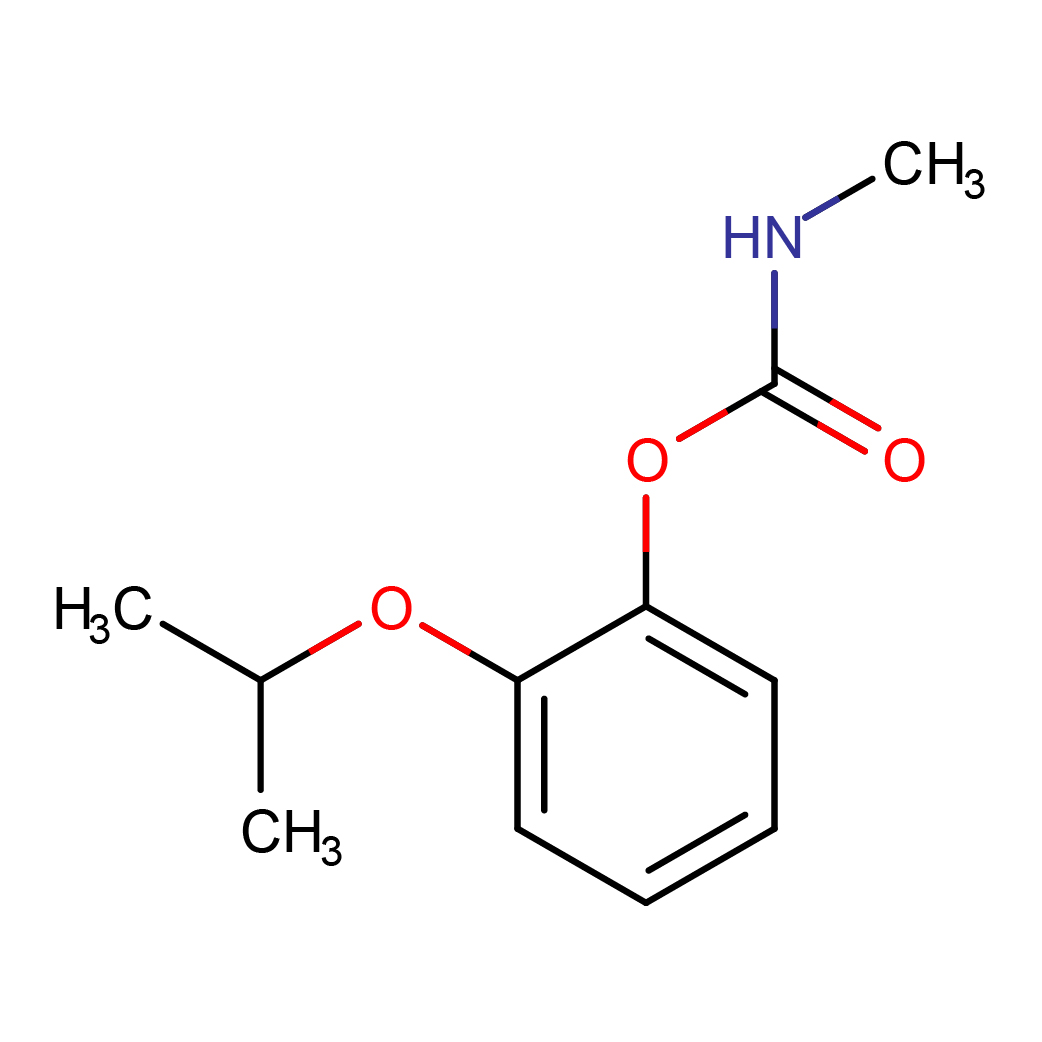
3D-structure