4-tert-octylphenol
Synonyms: "p-tert-octylphenol", "4-t-octylphenol", "4-(1,1,3,3-tetramethylbutyl)phenol,"para-tert-octylphenol"
Source: 4-tert-octylphenol is used in production of phenol/formaldehyde resins.
Identifiers:
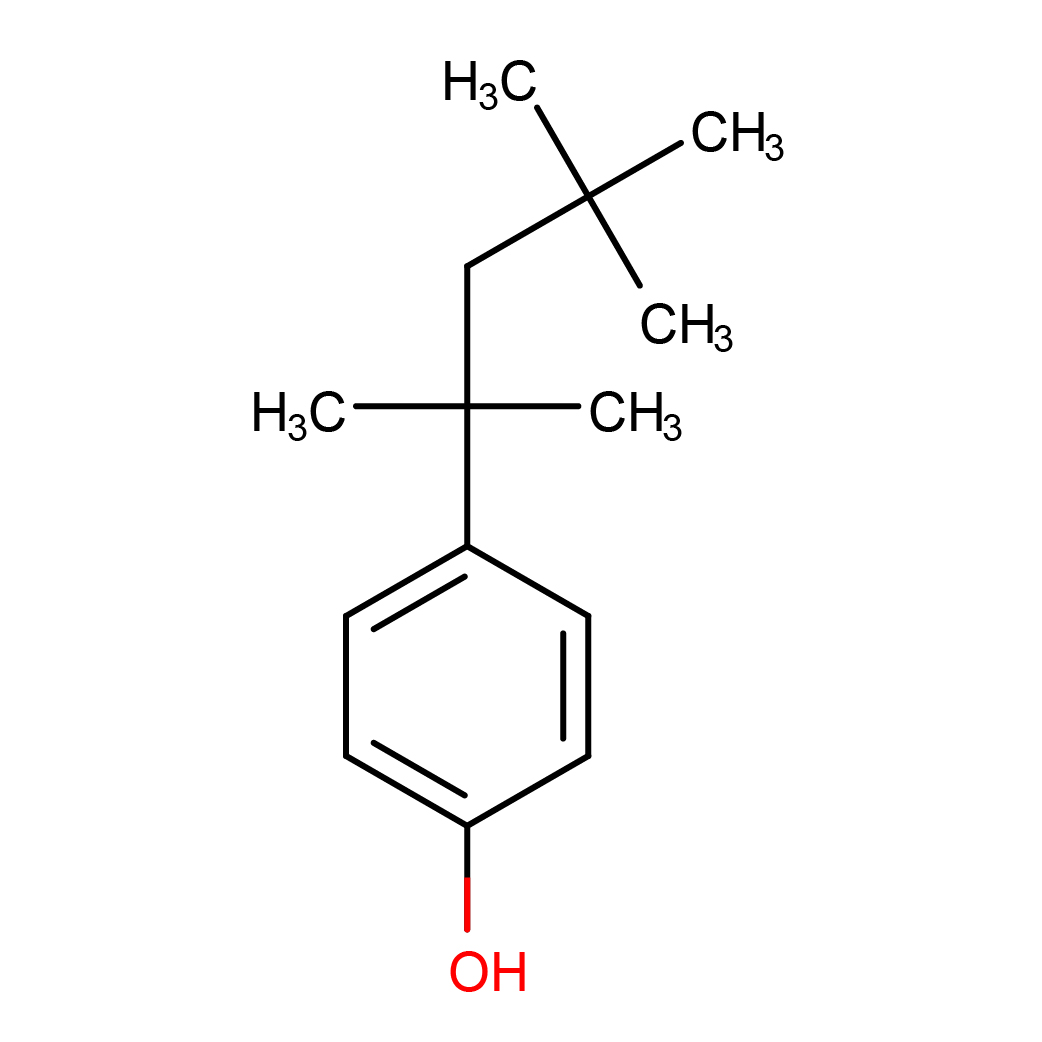
IUPAC Name: 4-(2,4,4-trimethylpentan-2-yl)phenol
CAS Number: 140-66-9
PubChem ID: 8814
InChiKey: ISAVYTVYFVQUDY-UHFFFAOYSA-N
Canonical SMILES: CC(C)(C)CC(C)(C)C1=CC=C(C=C1)O
Structural Properties:
Molecular Formula: C14H22O
Molecular Weight: 206.324
Pharmacophore Features:
Number of bond donors: 1
Number of bond acceptors: 1
Number of atoms different from hydrogen: 15
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Abraham EJ, Frawley LS. 1997. Octylphenol (OP), an environmental estrogen, stimulates prolactin (PRL) gene expression. Life Sci 60(17):1457-1465.
Boockfor FR, Blake CA. 1997. Chronic administration of 4-tert-octylphenol to adult male rats causes shrinkage of the testes and male accessory sex organs, disrupts spermatogenesis, and increases the incidence of sperm deformities. Biol Reprod 57(2):267-277.
Jobling S, Sheahan D, Osborne JA, Matthiessen P, Sumpter JP. 1996. Inhibition of testicular growth in rainbow trout (Oncorhynchus mykiss) exposed to estrogenic alkylphenolic chemicals. Environ Toxicol Chem 15(2):194-202.
Nishihara T, Nishikawa J, Kanayama T, Dakeyama F, Saito K, Imagawa M, Takatori S, Kitagawa Y, Hori S, Utsumi H. 2000. Estrogenic activities of 517 chemicals by yeast two-hybrid assay. Journal of Health Science 46(4):282-298.
Tran DQ, Klotz DM, Ladlie BL, Ide CF, McLachlan JA, Arnold SF. 1996. Inhibition of progesterone receptor activity in yeast by synthetic chemicals. Biochem Biophys Res Commun 229(2):518-523.
External Links


2D-structure
3D-structure