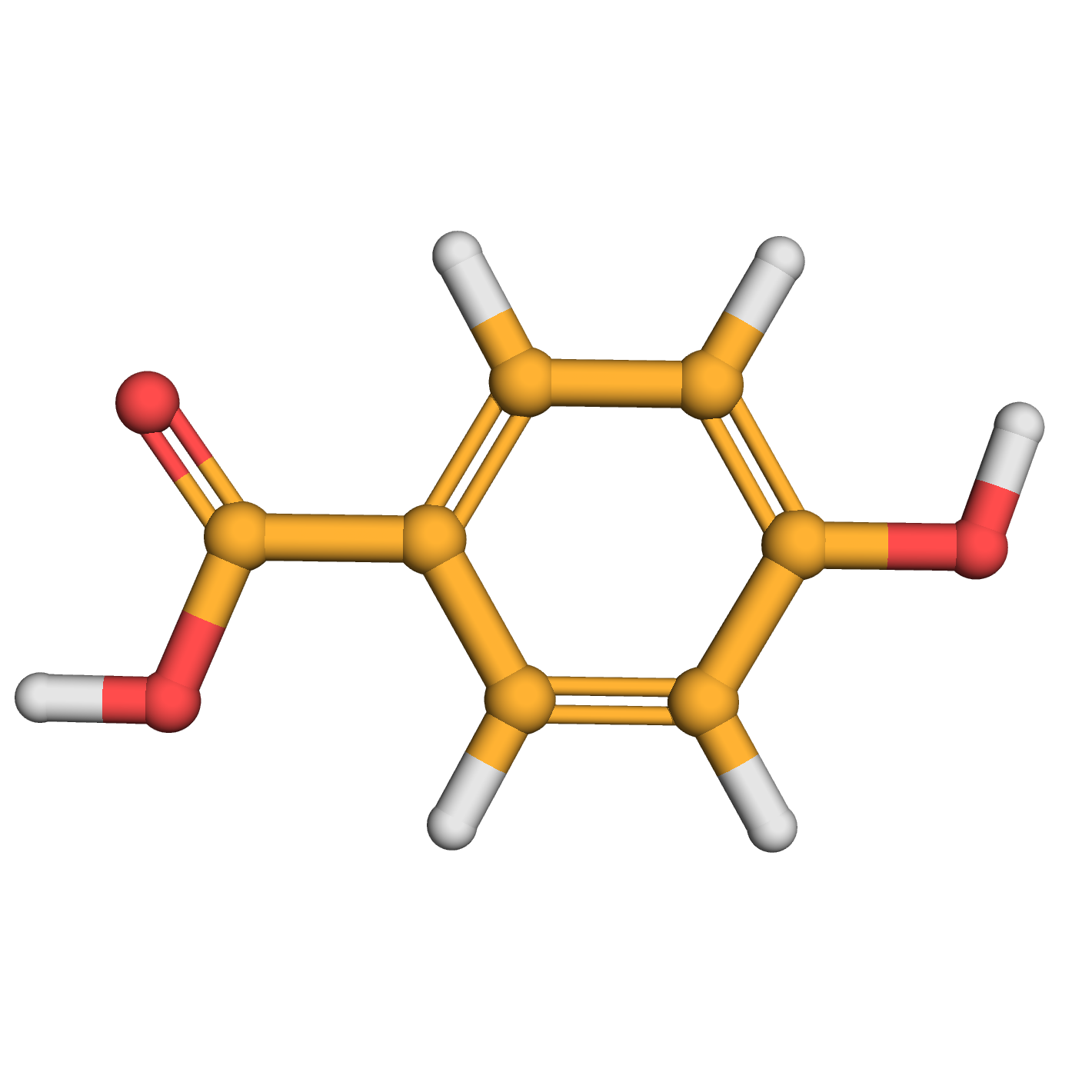
p-hydroxybenzoic acid
Synonyms: "4-hydroxybenzoic acid", "4-carboxyphenol", "p-salicylic acid", "p-carboxyphenol", "para-hydroxybenzoic acid", "p-hydroxybenzoic acid"
Source: 4-hydroxybenzoic acid is used to derive paraben, a common preservative used in the cosmetic industry.
Identifiers:
IUPAC Name: 4-hydroxybenzoic acid
CAS Number: 99-96-7
PubChem ID: 135
InChiKey: FJKROLUGYXJWQN-UHFFFAOYSA-N
Canonical SMILES: C1=CC(=CC=C1C(=O)O)O
Structural Properties:
Molecular Formula: C7H6O3
Molecular Weight: 138.121
Pharmacophore Features:
Number of bond donors: 2
Number of bond acceptors: 3
Number of atoms different from hydrogen: 10
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Lemini C, Silva G, Timossi C, Luque D, Valverde A, Gonzá,lez-Martí,nez M, Hernandez A, Rubio-Pó,o C, Chá,vez Lara B, Valenzuela F. 1997. Estrogenic effects of p-hydroxybenzoic acid in CD1 mice. Environ Res 75(2):130-134.
Peungvicha P, Thirawarapan SS, Watanabe H. 1998. Possible mechanism of hypoglycemic effect of 4-hydroxybenzoic acid, a constituent of Pandanus odorus root. Jpn J Pharmacol 78(3):395-398.
Pugazhendhi D, Pope GS, Darbre PD. 2005. Oestrogenic activity of p-hydroxybenzoic acid (common metabolite of paraben esters) and methylparaben in human breast cancer cell lines. J Appl Toxicol 25(4):301-319.
External Links

2D-structure
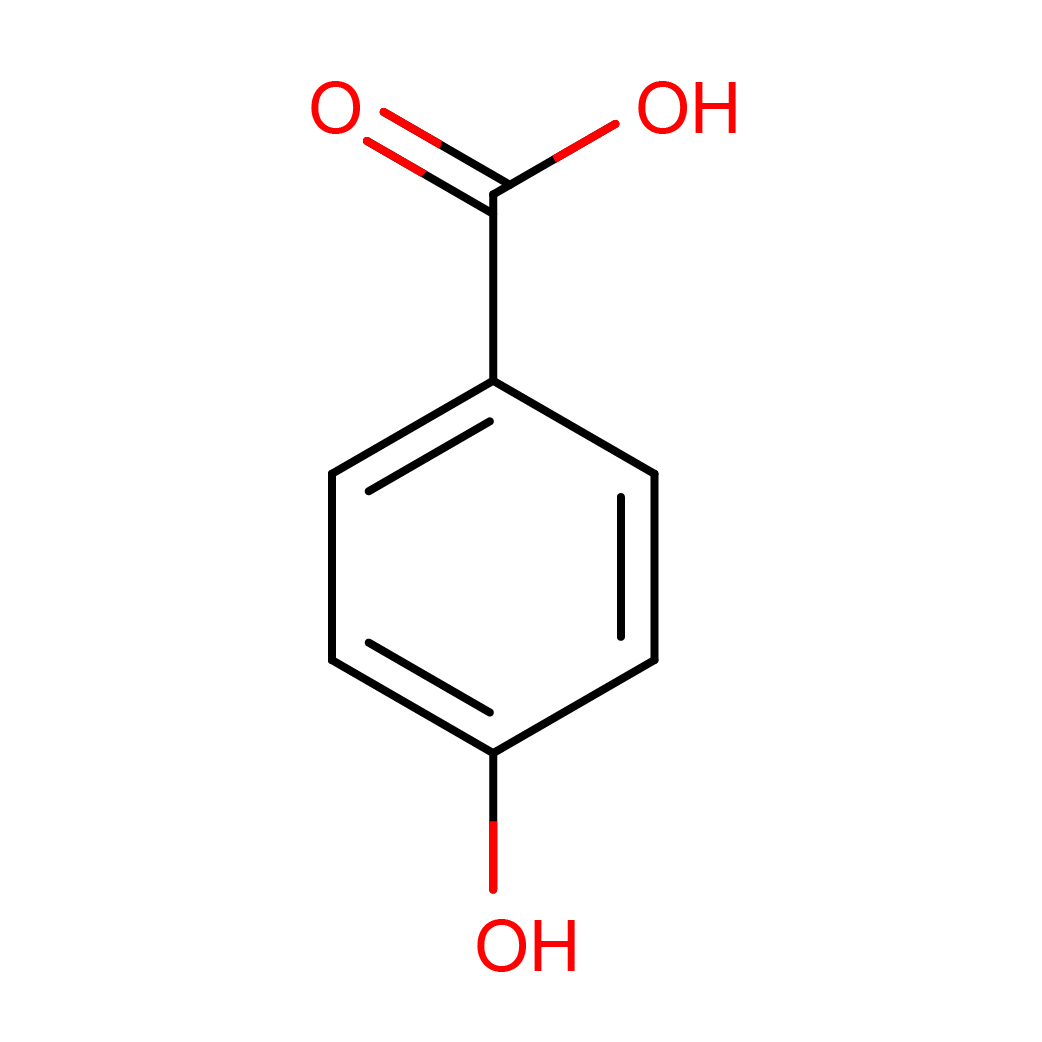
3D-structure