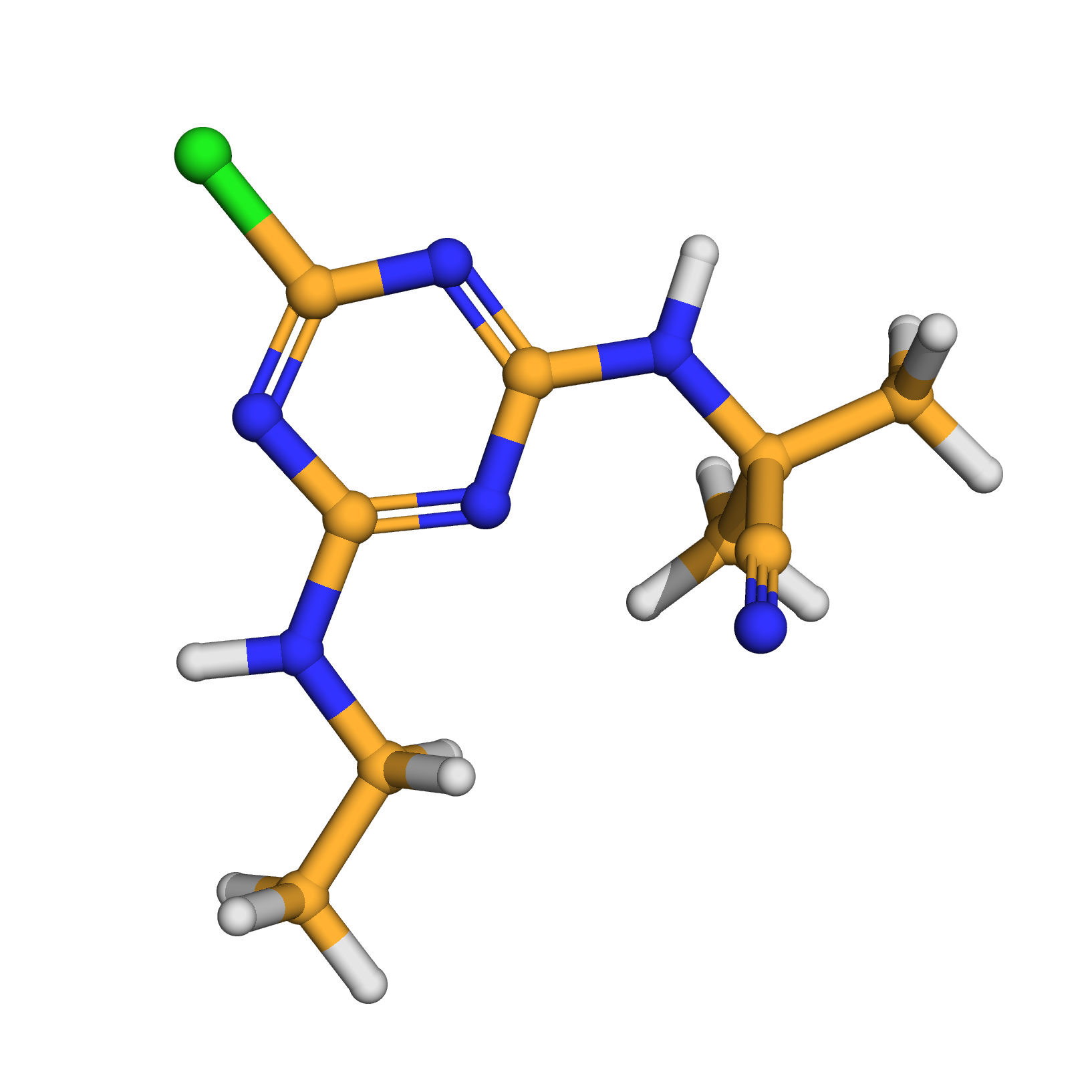
cyanazine
Synonyms: "fortrol", "bladex", "bladex 80WP", "cyanazin", "payze", "blanchol"
Source: cyanazine is a member of the s-triazine chemical family and is used as a herbicide for pre- and post-emergent to control annual grasses and broadleaf weeds.
Identifiers:
IUPAC Name: 2-[[4-chloro-6-(ethylamino)-1,3,5-triazin-2-yl]amino]-2-methylpropanenitrile
CAS Number: 21725-46-2
PubChem ID: 30773
InChiKey: MZZBPDKVEFVLFF-UHFFFAOYSA-N
Canonical SMILES: CCNC1=NC(=NC(=N1)Cl)NC(C)(C)C#N
Structural Properties:
Molecular Formula: C9H13ClN6
Molecular Weight: 240.693
Pharmacophore Features:
Number of bond donors: 2
Number of bond acceptors: 6
Number of atoms different from hydrogen: 16
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Tran DQ, Kow KY, McLachlan JA, Arnold SF. 1996. The inhibition of estrogen receptor-mediated responses by chloro-s-triazine-derived compounds is dependent on estradiol concentration in yeast. Biochemical & Biophysical Research Communications 227(1):140-146.
Vonier PM, Crain DA, McLachlan JA, Guillette LJ Jr., Arnold SF. 1996. Interaction of environmental chemicals with the estrogen and progesterone receptors from the oviduct of the American alligator. Environ Health Perspect 104(12):1318-1322.
External Links


2D-structure
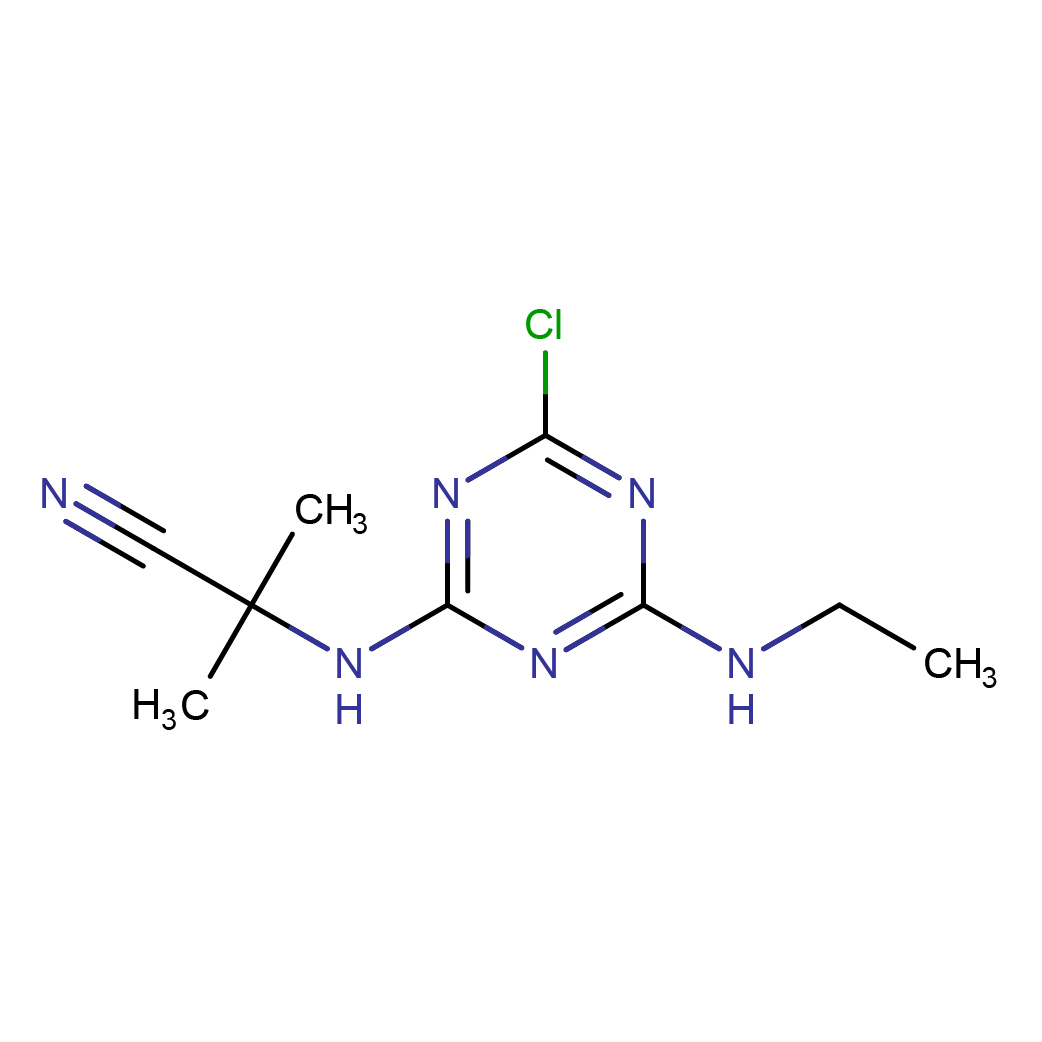
3D-structure