naringenin
Synonyms: "naringenine", "4',5,7-trihydroxyflavanone", "5,7-dihydroxy-2-(4-hydroxyphenyl)chroman-4-one"
Source: naringenin is a flavonoid found in grapefruit.
Identifiers:
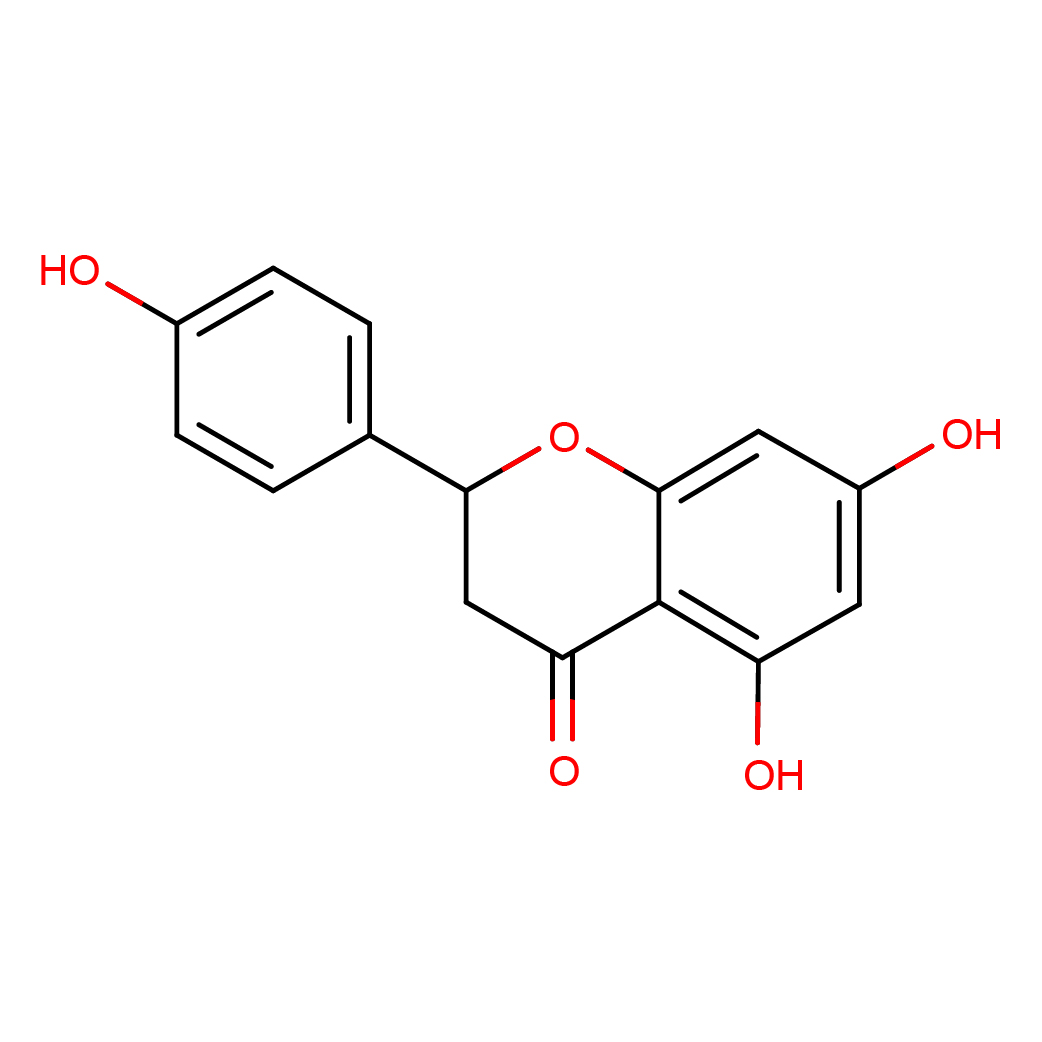
IUPAC Name: 5,7-dihydroxy-2-(4-hydroxyphenyl)-2,3-dihydrochromen-4-one
CAS Number: 480-41-1
PubChem ID: 932
InChiKey: FTVWIRXFELQLPI-UHFFFAOYSA-N
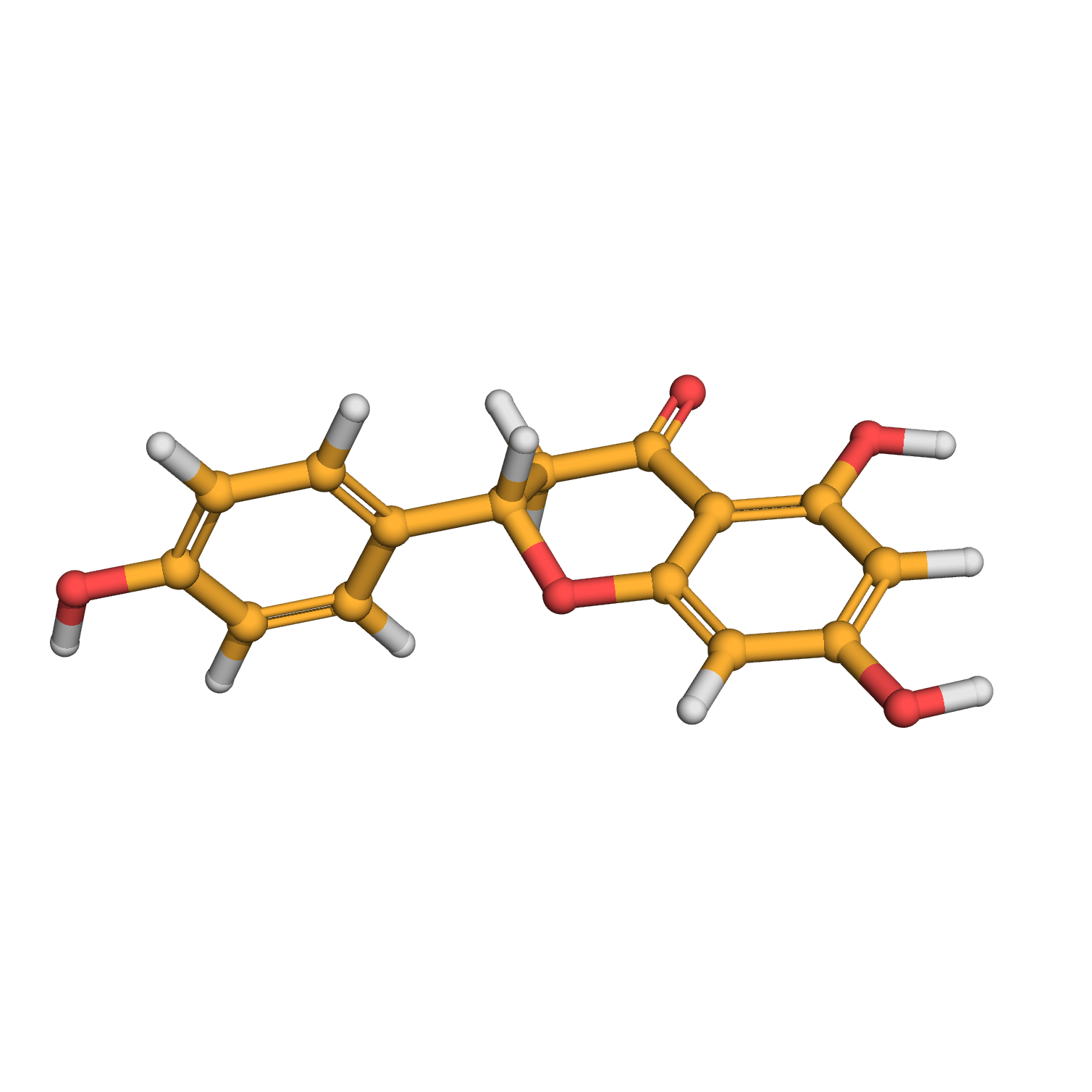
Canonical SMILES: C1C(OC2=CC(=CC(=C2C1=O)O)O)C3=CC=C(C=C3)O
Structural Properties:
Molecular Formula: C15H12O5
Molecular Weight: 272.253
Pharmacophore Features:
Number of bond donors: 3
Number of bond acceptors: 5
Number of atoms different from hydrogen: 20
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Kuiper GGJM, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg P, Gustafsson JA. 1998. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 139(10):4252-4263.
Nishihara T, Nishikawa J, Kanayama T, Dakeyama F, Saito K, Imagawa M, Takatori S, Kitagawa Y, Hori S, Utsumi H. 2000. Estrogenic activities of 517 chemicals by yeast two-hybrid assay. Journal of Health Science 46(4):282-298.
Rosenberg RS, Grass L, Jenkins DJ, Kendall CWC, Diamandis EP. 1998. Modulation of androgen and progesterone receptors by phytochemicals in breast cancer cell lines. Biochemical & Biophysical Research Communications 248(3):935-939.
External Links


2D-structure
3D-structure