monohydroxymethoxychlor
Synonyms: "1,1,1-trichloro-2-(4-hydroxyphenyl)-2-(4-methoxyphenyl)ethane ", "4-[2,2,2-trichloro-1-(4-methoxyphenyl)ethyl]phenol"
Source: monohydroxymethoxychlor is a derivative of the insecticide methoxychlor.
Identifiers:
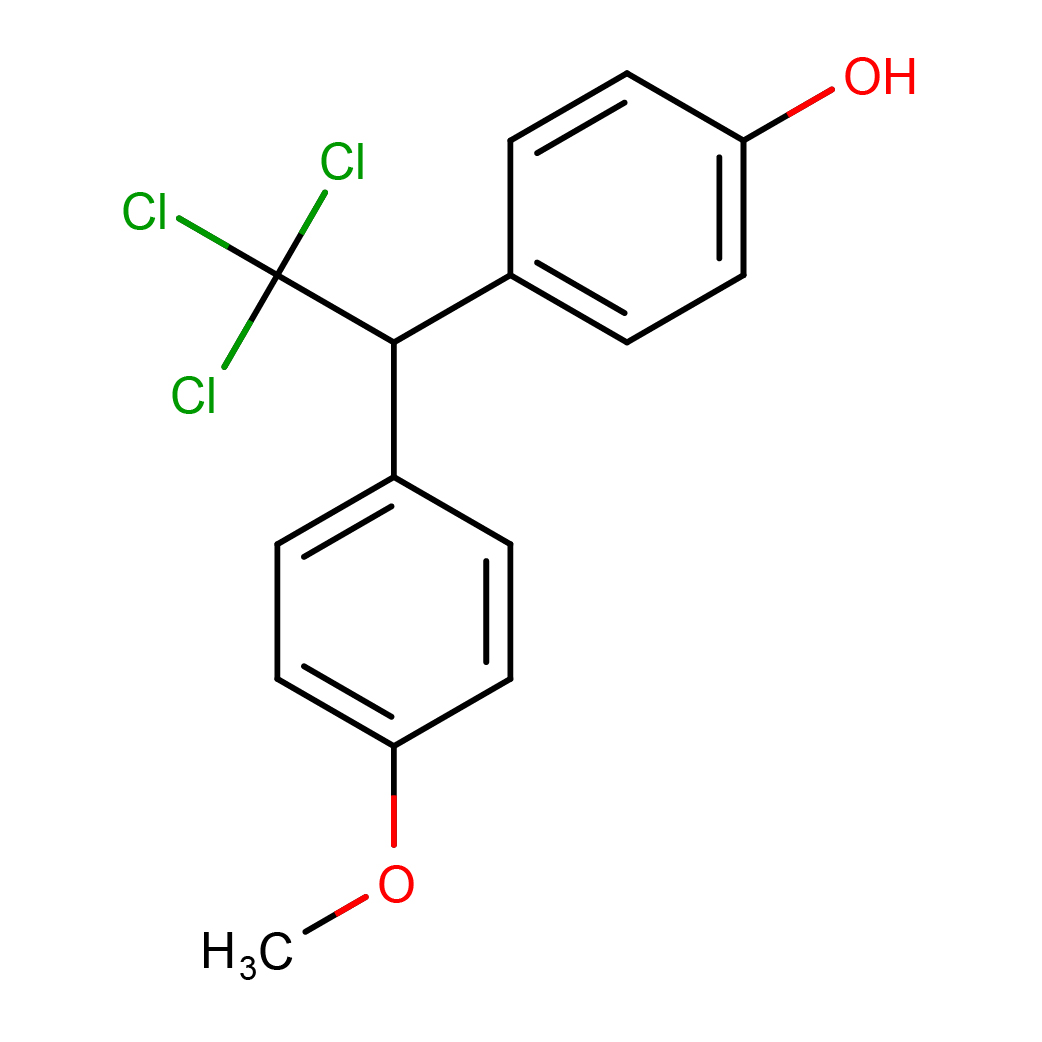
IUPAC Name: 4-[2,2,2-trichloro-1-(4-methoxyphenyl)ethyl]phenol
CAS Number: 28463-03-8
PubChem ID: 183679
InChiKey: BUZUQNXGKRJIJT-UHFFFAOYSA-N
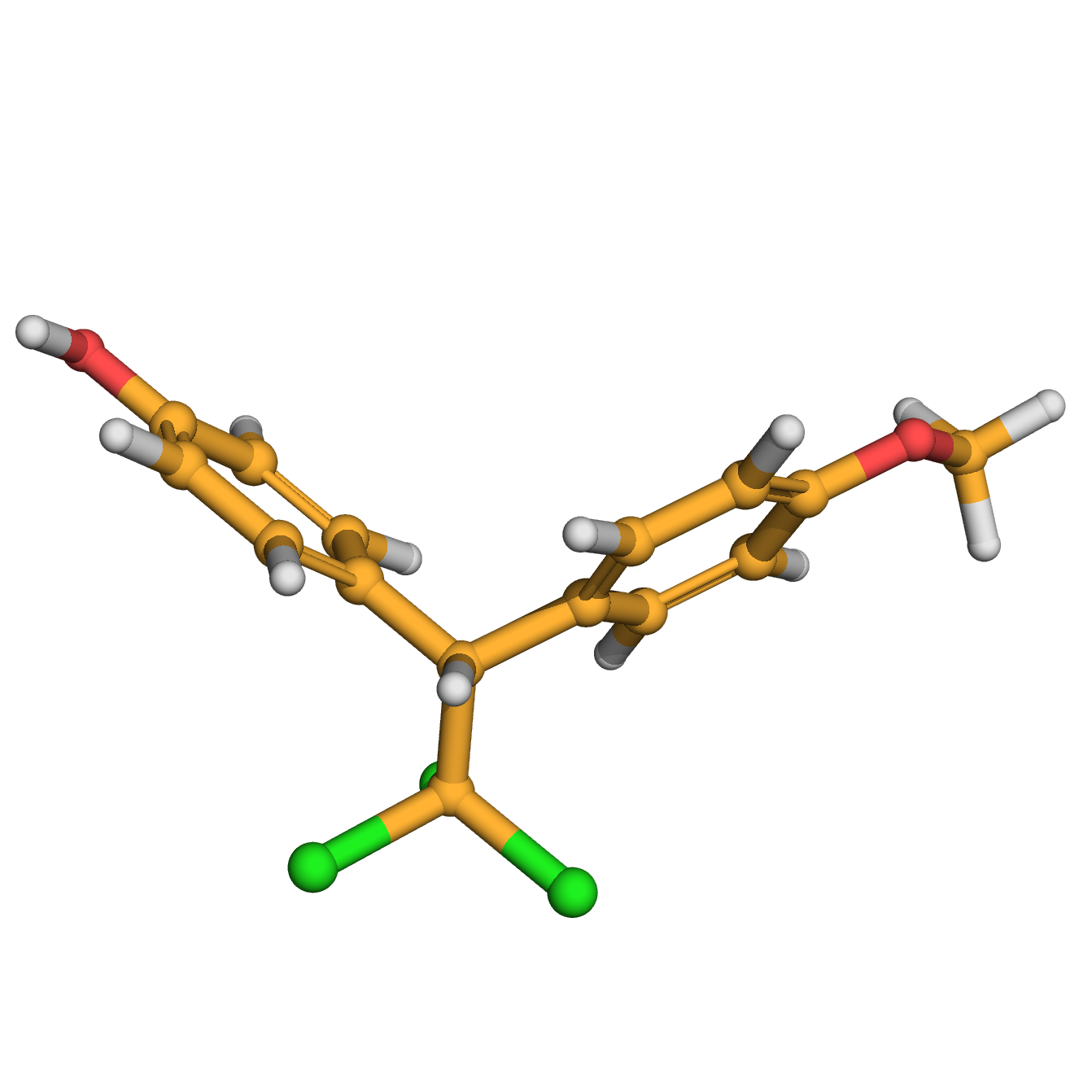
Canonical SMILES: COC1=CC=C(C=C1)C(C2=CC=C(C=C2)O)C(Cl)(Cl)Cl
Structural Properties:
Molecular Formula: C15H13Cl3O2
Molecular Weight: 331.622
Pharmacophore Features:
Number of bond donors: 1
Number of bond acceptors: 2
Number of atoms different from hydrogen: 20
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Blair RM, Fang H, Branham WS, Hass BS, Dial SL, Moland CL, Tong W, Shi L, Perkins R, Sheehan DM. 2000. The estrogen receptor relative binding affinities of 188 natural and xenochemicals: structural diversity of ligands. Toxicol Sci 54(1):138-153.
Gaido KW, Maness SC, McDonnell DP, Dehal SS, Kupfer D, Safe S. 2000. Interaction of methoxychlor and related compounds with estrogen receptor [alpha] and [beta], and androgen receptor: structure-activity studies. Mol Pharmacol 58(4):852-858.
Ousterhout J, Struck RF, Nelson JA. 1981. Estrogenic activities of methoxychlor metabolites. Biochem Pharmacol 30(20):2869-2871.
External Links

2D-structure
3D-structure