bisphenol AF
Synonyms: "4,4'-(hexafluoroisopropylidene)diphenol", "2,2-bis(4-hydroxyphenyl)hexafluoropropane", "hexafluorobisphenol a", "hexafluorodiphenylolpropane"
Source: bisphenol AF is used to cross-link and cure fluoroelastomers, often along with an accelerator such as triphenyl benzyl phosphonium chloride. When used as a curing agent it produces elastomers with excellent chemical resistance and thermal stability. Bisphenol AF is also used as a monomer in the synthesis of specialty polymers. Polymers containing Bisphenol AF are useful in high temperature composites, in electronic materials and other specialty applications.
Identifiers:
IUPAC Name: 4-[1,1,1,3,3,3-hexafluoro-2-(4-hydroxyphenyl)propan-2-yl]phenol
CAS Number: 1478-61-1
PubChem ID: 73864
InChiKey: ZFVMWEVVKGLCIJ-UHFFFAOYSA-N
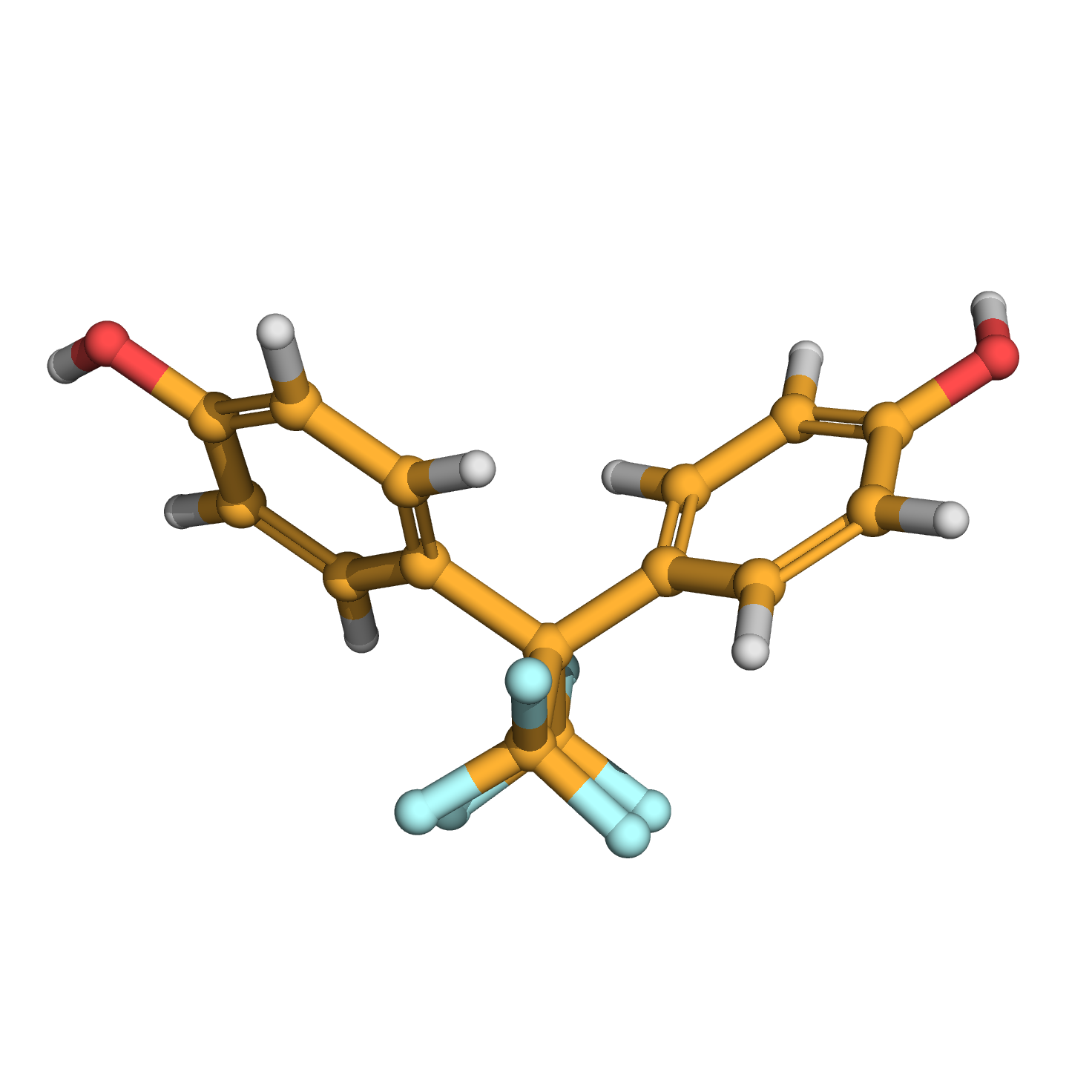
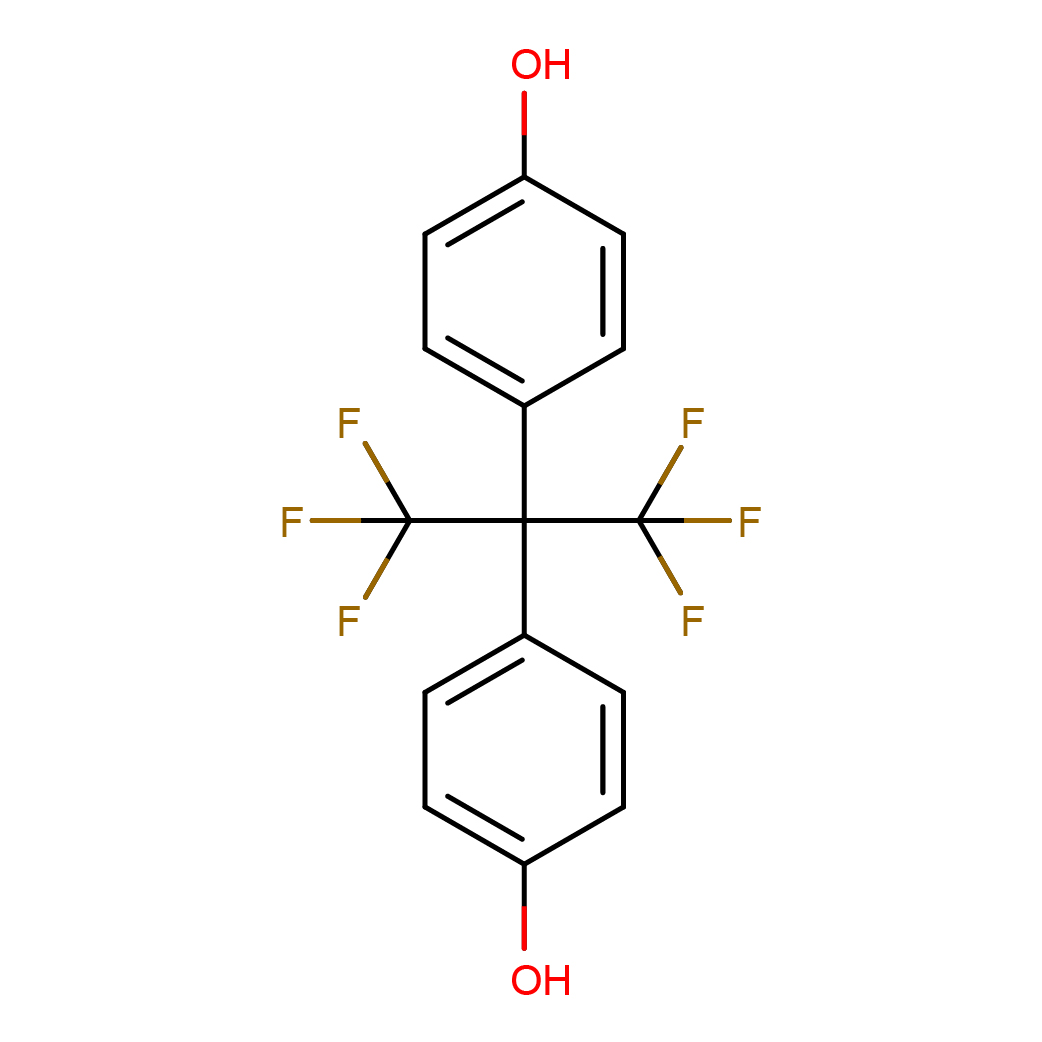
Canonical SMILES: C1=CC(=CC=C1C(C2=CC=C(C=C2)O)(C(F)(F)F)C(F)(F)F)O
Structural Properties:
Molecular Formula: C15H10F6O2
Molecular Weight: 336.229
Pharmacophore Features:
Number of bond donors: 2
Number of bond acceptors: 2
Number of atoms different from hydrogen: 23
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Akahori Y, Nakai M, Yamasaki K, Takatsuki M, Shimohigashi Y, Ohtaki M. 2008. Relationship between the results of in vitro receptor binding assay to human estrogen receptor alpha and in vivo uterotrophic assay: Comparative study with 65 selected chemicals. Toxicol in Vitro 22(1):225-231.
Perez P, Pulgar R, Olea-Serrano F, Villalobos M, Rivas A, Metzler M, Pedraza V, Olea N. 1998. The estrogenicity of bisphenol A-related diphenylalkanes with various substituents at the central carbon and the hydroxy groups. Environ Health Perspect 106(3):167-174.
External Links

2D-structure
3D-structure